Three-dimensional NiCo2O4/NiCo2S4 hybrid nanostructure on Ni-foam as a high-performance supercapacitor electrode†
Shipra Raja,
Suneel Kumar Srivastavab,
Pradip Kara and
Poulomi Roy*a
aDepartment of Chemistry, Birla Institute of Technology Mesra, Ranchi 835215, Jharkhand, India. E-mail: poulomiroy@yahoo.com
bDepartment of Chemistry, Indian Institute of Technology Kharagpur, Kharagpur 721302, West Bengal, India
First published on 22nd September 2016
Abstract
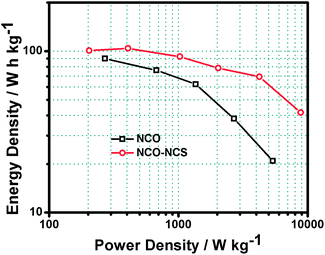
The spinel structured ternary mixed metal oxide NiCo2O4 and sulphide NiCo2S4 are considered as promising pseudocapacitive materials. In view of this, the present work involves the fabrication of NiCo2O4 nanosheets and a NiCo2O4/NiCo2S4 hybrid nanostructure on Ni-foam as a conductive substrate by a facile ammonia evaporation technique. The NiCo2O4/NiCo2S4 hybrid nanostructure exhibits remarkable supercapacitive performance compared to bare NiCo2O4 nanosheets due to its three dimensional open structure and synergistic effect of NiCo2O4 and NiCo2S4. The hybrid nanostructure exhibits a specific capacitance as high as 3671 F g−1 at a current density of 1.8 A g−1 and 2767 F g−1 at 9 A g−1 with a capacity retention value of 84% at 10 A g−1 after 2000 cycles. Furthermore, the electrode composed of the NiCo2O4/NiCo2S4 hybrid nanostructure displays a noticeably high energy density as well as power density (8820 W kg−1 at 41.65 W h kg−1) compared to the NiCo2O4 nanosheets (5374.28 W kg−1 at 20.90 W h kg−1) with great flexibility. It is anticipated that the combination of materials and the special structural design lead to fast electrochemical redox reactions, fast electron/ionic transportation and mechanical integrity, which promote the NiCo2O4/NiCo2S4 hybrid nanostructure as a superior electrode in high performing flexible supercapacitors.
1. Introduction
Harnessing energy has been receiving considerable amount of attention in recent years due to its ever-increasing demand and the limited availability of fossil fuels. Although the availability of ecofriendly renewable energy sources helps to avoid the necessity of fossil fuels, energy storage remains equally important in order to overcome the unpredictable nature of renewable energy sources.1–4 In view of this, supercapacitors are considered as the most potential energy storage devices due to their very fast charging–discharging process, longer life span and high power density.3,5–7 Although carbon-based electrodes are well-known for this purpose, their low specific as well as volumetric capacitance values limit their extensive use in supercapacitors.8,9 Various binary transition metal oxides, such as, MnO2,10,11 NiO,12–14 Co3O4,15,16 and Fe3O4,17,18 have been used as alternative materials. In addition, spinel structured mixed transition metal oxides (MTMOs) and sulphides (MTMSs) have attracted much attention as promising pseudocapacitive materials due to their good electrical conductivity and availability of multiple metal oxidation states to facilitate redox reactions.19–29 According to the available literature, spinel NiCo2S4 shows ∼100 times higher electrical conductivity compared to NiCo2O4.23,30 In another study, almost 2.5 times improvement in the electrochemical performance of the supercapacitor with a high specific capacitance of 1501 F g−1 at current density 1 A g−1 was reported for porous NiCo2S4 nanonetworks than that of NiCo2O4 nanosheets.31The reduction of size to the nanoscale is considered to be an additional aspect exhibited by superior charge storage systems having large surface areas compared to their bulk counterparts.2,4 The anisotropic growth of nanostructures in the form of nanorods, nanowires, and nanoflakes on conductive substrates has drawn much interest due to faster electron transportation rates and better mechanical integrity against the repeated charging–discharging process.4 As a result, various synthetic procedures have been adopted to fabricate such binder-free nanostructures directly grown onto a conductive substrate. Among them, the hydro/solvothermal method is the most studied method for the synthesis of mixed metal oxide or sulfide nanostructures.21,24–26,31–43 Some reports are available on the deposition of spinel MTMOs and MTMSs nanoflakes or nanowire arrays on Ni foam via general solution based methods under moderate reaction temperatures (90–100 °C).27,44,45 Further advanced hybrid nanostructures combining more than one MTMOs or MTMSs on conductive substrates contribute significant supercapacitive performances due to the synergistic effect.21,25,26,32 Cheng and co-workers25 fabricated hierarchical core–shell NiCo2O4@NiCo2O4 nanocactus arrays by a hydrothermal followed by electrodeposition method. These core–shell designed NiCo2O4@NiCo2O4 showed good electrochemical performances in a supercapacitor with the specific capacitance of 1264 F g−1 at a current density of 2 A g−1. Liu et al.21 synthesized NiCo2O4@NiCo2O4 core–shell nanoflake arrays on Ni-foam by a hydrothermal followed by chemical bath deposition method. The electrode showed a specific capacitance of 1115 F g−1 at a current density 5 mA cm−2. In another study, NiCo2O4@NiMoO4 hybrid core–shell nanowire/nanosheet arrays were fabricated on Ni foam by a two-step hydrothermal method followed by calcination.26 The optimized NiCo2O4@NiMoO4 hybrid electrode exhibited the specific capacitance of up to 1261 F g−1 at the current density of 10 A g−1. Various dendritic heterojunction nanowire arrays with NiCo2S4 nanowires as cores and NiCo2O4, NiO, Co3O4, and MnO2 nanowires as branches on Ni foam were fabricated by a two-step hydrothermal treatment, as reported by Zou et al.32 The electrodes exhibited excellent areal capacitances of 10.99 F cm−2, 6.83 F cm−2, 7.47 F cm−2 and 5.9 F cm−2 at current density of 10 mA cm−2 for NiCo2S4/MnO2, NiCo2S4/NiO, NiCo2S4/Co3O4, and NiCo2S4/NiCo2O4, respectively.
In the present report, three-dimensional novel NiCo2O4/NiCo2S4 hybrid nanostructures are deposited on Ni-foam by a two-step pH-controlled ammonia evaporation technique and post-calcination process. These hybrid nanostructures exhibit remarkable electrochemical performances compared to pristine NiCo2O4 nanostructures on Ni-foam for use as a supercapacitor. To the best of our knowledge, this is the highest specific capacitance value achieved thus far with this combination of materials and nanostructure design. The increased active sites in the nanostructures, redox-rich surface availability and the synergistic effects of both NiCo2O4 and NiCo2S4 in the hybrid nanostructure account for the high supercapacitive performances.
2. Experimental
2.1 Materials synthesis
All the chemicals used in our experiments were of analytical grade and were used without further purification. In a typical procedure, 6 mM of nickel(II) chloride hexahydrate (Sigma-Aldrich, ≥98%) and 12 mM cobalt(II) acetate tetrahydrate (Sigma-Aldrich, ≥98.0%) were mixed together in 15 mL deionized water followed by the addition of an ammonia solution (25%) under stirring and maintenance of a pH value of 12. The Ni-foam was treated with 6 M HCl under sonication for 30 min to remove the native oxide layer, and cleaned with distilled water, ethanol and acetone. The pre-treated Ni-foam was then placed into a beaker and kept in an oven at 90 °C for the slow evaporation of ammonia. After 20 h, the Ni-foam was collected, and cooled down to room temperature naturally. The sample was then sonicated in distilled water for 5 min and dried in an oven at 50 °C. Multiple depositions were carried out in order to increase the mass loading of sample onto the Ni-foam. The as deposited precursor material was then calcined at 350 °C for 2 h in air for conversion into NiCo2O4 deposited on Ni-foam.In the 2nd step, a similar process in the presence of 40 mM thiourea (Merck, India) was carried out to deposit NiCo2S4 on the pre-deposited NiCo2O4 on Ni-foam to obtain the NiCo2O4/NiCo2S4 hybrid material. The as synthesized hybrid material was then sonicated in distilled water for 5 min to remove loosely adhered particles and finally dried in an oven at 50 °C.
2.2 Structural characterization
X-ray diffraction (XRD) patterns of the materials were recorded on a Philips PW-1710 X-ray diffractometer (40 kV, 20 mA) using Cu Kα radiation (λ = 1.5418 Å) in the 2θ range of 20–60°. X-ray photoelectron spectroscopy (XPS) was performed on a PHI 5000 Versa Probe II (ULVAC-PHI, INC, Japan) system using a microfocused (100 μm, 25 W, 15 kV) monochromatic Al Kα source (hν = 1486.6 eV), a hemispherical analyzer, and a multichannel detector. The typical vacuum in the analysis chamber during the measurements was in the range of 1 × 10−10 Torr. Charge neutralization was used for all measurements using a combination of low energy Ar+ ions and electrons. The binding energy scale was charge referenced to the C 1s at 284.6 eV. The morphology of the samples was characterized by scanning electron microscopy (SEM, JEOL JSM-6390LV) and the compositions of the samples were determined by energy-dispersive X-ray analysis (EDX). Further morphological analysis of the samples by high-resolution transmission electron microscopy (HR-TEM) was performed on a TECNAI G2, SEI (Netherland) operating at 200 kV and a Gatan multipole charge coupled device (CCD) camera. Prior to the analysis, the samples were prepared by drop casting on carbon-coated copper grids and drying overnight under vacuum. Nitrogen adsorption/desorption measurements were performed to investigate the surface characteristics at 77 K using a surface area analyzer (Quantachrome Autosorb iQ).2.3 Electrochemical measurement
The capacitance performances of samples were determined with an AUTOLAB electrochemical workstation (AUTOLAB 302N) using the three-electrode configuration. The binder-free sample deposited on Ni-foam itself was used as the working electrode, Pt as the counter electrode and Ag/AgCl as the reference electrode. Cyclic voltammetry (CV), the charge–discharge experiment and electrochemical impedance spectroscopy (EIS) analysis were carried out using 1 M aqueous KOH solution as the electrolyte.3. Results and discussion
3.1 Reaction mechanism
The deposition of NiCo2O4 nanosheets on Ni-foam was carried out by a pH controlled ammonia evaporation technique. Earlier the technique was used for synthesizing various binary metal oxides and hydroxides.46,47 However, to the best of our knowledge, there are no reports are available on the preparation of MTMOs or MTMSs by this technique. The pH of solution plays a very important role for the deposition of materials. In presence of excess ammonia, both Ni2+ and Co2+ form the stable complexes Ni(NH3)62+ and Co(NH3)62+ according to eqn (1) and (2). During the progress of the reaction at 90 °C, ammonia slowly evaporates and the pH of the solution decreases, which initiates the hydrolysis of the ammonia complexes, as represented in eqn (3), and leads to the deposition of mixed metal hydroxide precursors on the Ni-foam. Finally, the as-synthesized NiCo-hydroxide nanosheets are converted into NiCo2O4 nanosheets on Ni foam by the calcination process in air (eqn (4)).| Ni2+ + 6NH3 → Ni(NH3)62+ | (1) |
| Co2+ + 6NH3 → Co(NH3)62+ | (2) |
 | (3) |
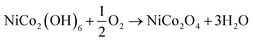 | (4) |
The growth of the NiCo-hydroxide nanosheets at different reaction durations with a continuous change in pH was investigated thoroughly and represented in Fig. S1.† Our study shows that the deposition of the hydroxide precursor starts after 3 h of reaction progress when the pH of solution reaches 11 and the growth continues with a continuous decrease in the pH value of solution. However, very negligible deposition was observed when the reaction was carried out by adding a few drops of ammonia to maintain the pH at 11 (Fig. S2†). It is believed that while ammonia controls both the precipitation and passivation of selective surfaces, the pH of solution controls the charges of the particles influencing the growth of the nanosheets.46 The presence of excess ammonia in solution stabilizes the particles formed at the initial stage of the reaction by capping on the particle surface and on continuing the reaction, the pH decreases and the particles are un-capped slowly, which leads to growth in a specific direction. The influence of ammonia concentration on the growth of the NiCo-hydroxide nanosheets was also investigated and shown in Fig. S3.† It is observed that highly dense, interconnected, sharp edged nanosheets were formed when the deposition was carried out in 25% (50 mL) ammonia solution. However, on increasing the water content, keeping the reaction duration constant, the density of the nanosheets decreased and at the ammonia![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio of 20
water ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, the underneath Ni-foam was exposed. Furthermore, the mass loading of materials on the Ni-foam can be controlled by multiple depositions, as shown in Fig. 1, which result in thickening of the nanosheets (Fig. S4†).
30, the underneath Ni-foam was exposed. Furthermore, the mass loading of materials on the Ni-foam can be controlled by multiple depositions, as shown in Fig. 1, which result in thickening of the nanosheets (Fig. S4†).
The deposition of NiCo2S4 was carried out in a similar way using thiourea as the S-precursor. As the reaction proceeds and the pH of the solution is lowered, a reaction occurs between the NiCo-hydroxides and thiourea resulting in the deposition of NiCo2S4 nanoflake balls (Fig. S5†), as represented in eqn (5):
 | (5) |
The schematic diagram for the fabrication the 3D NiCo2O4/NiCo2S4 hybrid nanostructures on Ni foam is represented in Scheme 1. The NiCo2O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NiCo2S4 mass ratio of 3
NiCo2S4 mass ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the NCO–NCS hybrid material was maintained for the electrochemical analyses.
1 in the NCO–NCS hybrid material was maintained for the electrochemical analyses.
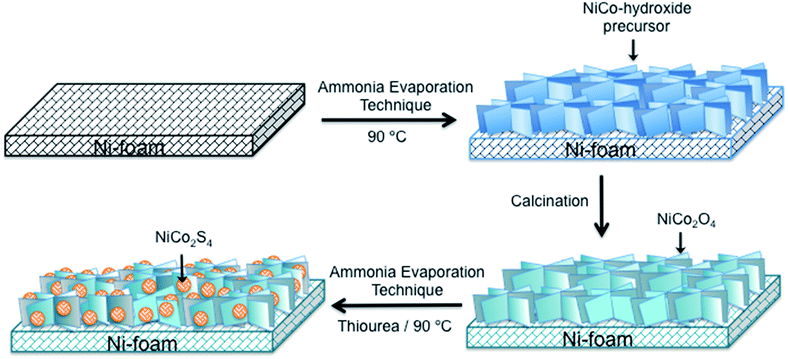 | ||
| Scheme 1 Schematic of the fabrication process of the NiCo2O4/NiCo2S4 hybrid nanostructure on Ni-foam. | ||
3.2 Characterization
The X-ray diffraction patterns of NiCo2O4 (NCO) and NiCo2S4 (NCS) deposited on Ni-foam were analyzed after multiple depositions and represented in Fig. 2. While most of the peaks in Fig. 2a indicate the formation of spinel NiCo2O4 (JCPDS no. 20-0781), Fig. 2b confirms the formation of NiCo2S4 (JCPDS no. 43-1477) on Ni-foam. The major peaks at 44.9° and 52.3° are indexed as Ni from the substrate. Although in the case of the NCO sample the absence of any other additional peak indicates the formation of NiCo2O4 only (Fig. S6†), and the XRD pattern of the NCS sample indicates the presence of a few small peaks assigned for NiO (JCPDS no. 78-0643) along with NiCo2S4 which originate from the partial oxidation of the Ni-foam during the NiCo2S4 deposition process. | ||
| Fig. 2 XRD pattern of NiCo2O4 (JCPDS no. 20-0781) nanosheets and NiCo2S4 (JCPDS no. 43-1477) nanoflakes deposited on Ni-foam (* indicates the presence of small peak of NiO). | ||
In order to gain further information about the elementary compositions of the nanostructures, X-ray photoelectron spectroscopy (XPS) analyses were carried out for NCO as well as the NCO–NCS samples and represented in Fig. 3. The high resolution Ni 2p and Co 2p spectra (Fig. 3a and b) for NiCo2O4 and NiCo2O4/NiCo2S4 can be fitted with two spin–orbit doublets characteristic of Ni2+/Ni3+ and Co2+/Co3+ and two shakeup satellites (Sat.) in each case by using the Gaussian fitting method.23,31 The percentages of Ni2+ and Ni3+ or Co3+ and Co2+ present in NCO and NCO–NCS were calculated based on the area of the deconvoluted peaks. The percentages of Ni2+ and Ni3+ are found to be 57% and 43% in NCO, and 59% and 41% in NCO–NCS, respectively. Moreover, the Co2+ and Co3+ percentages are found to be 50% and 50% in NCO, and 55% and 45% in NCO–NCS, respectively. These results are consistent with the previous results and the coexistence of Ni2+/Ni3+ and Co2+/Co3+ in both the samples provides abundant active sites for energy storage.48–50 The high resolution O 1s spectrum shows three different oxygen contributions which are denoted as O1, O2 and O3. The O1 at 529.5 eV is ascribed to the metal–oxygen bond, and the O2 at 531.2 eV is usually associated with defects, presence of surface species including hydroxyls, chemisorbed oxygen, or species intrinsic to the surface of the spinel.29 The comparatively small peaks situated at 532.6 eV correspond to the multiplicity of physi/chemisorbed water at the surface.29 The high resolution S 2p spectrum of the NiCo2O4/NiCo2S4 hybrid nanostructures shows the characteristic S 2p3/2 and S 2p1/2 at 161.7 eV and 163.3 eV, respectively, followed by a shakeup satellite at 168.1 eV.23
 | ||
| Fig. 3 XPS spectrum of NiCo2O4 nanosheets and NiCo2O4–NiCo2S4 hybrid nanostructures: (a) Ni 2p; (b) Co 2p; (c) O 1s and (d) S 2p. | ||
The morphology of the NiCo2O4 (NCO) nanosheets and NiCo2O4/NiCo2S4 (NCO–NCS) hybrid nanostructures on Ni foam was characterized by scanning electron microscope analyses. The SEM images of the NiCo2O4 nanosheets grown on Ni-foam by the ammonia evaporation technique are represented in Fig. 4. It is observed that the interconnected nanosheets with sharp edges are homogeneously grown on the Ni-foam and develop a uniform coating (Fig. 4a). The interconnected arrangements of nanosheets reveal flower-like structures with smaller nanoflake networks at the center and larger nanosheets organized as petals.
In the next step, NiCo2S4 nanoflake balls are deposited on the pre-deposited NiCo2O4 nanosheets on Ni-foam by the same technique in the presence of thiourea. Fig. 5 represents the SEM images of the NCO–NCS hybrid nanostructures fabricated on Ni foam by 3-times deposition of the NiCo-hydroxide precursor followed by calcination and one time deposition of NiCo2S4. It can be seen that the NiCo2S4 nanoflake balls are homogeneously distributed on the surfaces of the NiCo2O4 nanosheets grown on Ni-foam. A slight thickening of the NiCo2O4 nanosheets can be observed due to multiple depositions. The nanoflake structure of the NiCo2S4 balls can be noticed clearly, as shown in Fig. S5.†
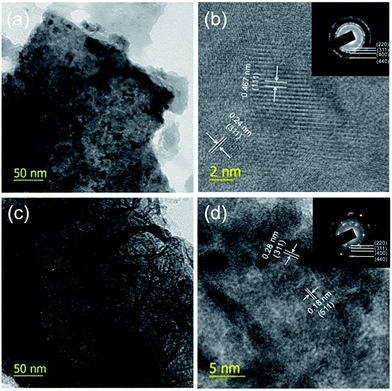
TEM analyses were carried out further to investigate the morphology of the NCO and NCO–NCS samples (Fig. 6). Fig. 6a shows the porous structure of the NiCo2O4 nanosheets and the lattice spacing of 0.467 nm and 0.24 nm in Fig. 6b correspond to the (111) and (311) planes of NiCo2O4, respectively. The selected-area electron diffraction (SAED) pattern of the NCO sample indicates its polycrystalline nature and reveals the clear rings assigned for the (220), (311), (400) and (440) planes of NiCo2O4. In the NCO–NCS samples, as shown in Fig. 6c and d, NiCo2S4 balls consisting of fine nanoflakes are clearly visible and their well-defined lattice spacing indicates the crystalline nature of the nanostructures. The SAED pattern of NiCo2S4 also reveals the appearance of clear rings corresponding to the (220), (311), (400) and (440) planes of NiCo2S4 (JCPDS no. 43-1477). The EDX pattern (Fig. S7†) shows the presence of Ni, Co, O and S with an Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio 1
Co ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in the NiCo2O4/NiCo2S4 nanohybrid material.
2 in the NiCo2O4/NiCo2S4 nanohybrid material.
The mesoporous nature of both NiCo2O4 and the NiCo2O4/NiCo2S4 hybrid material was investigated by BET measurement. The N2 adsorption–desorption isotherms of the samples shows the BET surface area of NiCo2O4 and NiCo2O4/NiCo2S4, which are calculated to be 81.89 m2 g−1 and 194.49 m2 g−1, along with the BJH pore size ranges of 6–17 nm and 6–11 nm, respectively (Fig. S8†). In accordance with the surface area, the BJH pore volumes of NCO and NCO–NCS are calculated as 0.211 and 0.253 cm3 g−1, respectively. The considerably higher surface area and narrow pore size distribution of the NiCo2O4/NiCo2S4 hybrid nanostructure favors easy ion access at the electrode/electrolyte interface, thus enhancing the faradaic reactions in comparison to the NiCo2O4 nanosheets.
In order to study the capacitive nature of the NCO and NCO–NCS samples, detailed investigations were carried out and compared. Fig. 7a and c show the cyclic voltammetric (CV) diagrams of NCO and NCO–NCS in the potential range of −0.2 V to 0.6 V at different scan rates between 5–100 mV s−1. The appearance of redox peaks indicates the pseudocapacitive nature of both samples. In NCO, the peaks can be attributed to Ni2+/Ni3+ and Co2+/Co3+/Co4+ involving the faradaic redox reactions of M–O/M–O–OH, as described in eqn (6) and (7).
| NiCo2O4 + OH− ↔ NiOOH + 2CoOOH + e− | (6) |
| CoOOH + OH− ↔ CoO2 + H2O + e− | (7) |
In the case of the NCO–NCS nanohybrid material, the presence of redox peaks are manifested for Ni2+/Ni3+ and Co2+/Co3+/Co4+ involving the M–S/M–S–OH and M–S–OH/M–S–O as well as the M–O/M–O–OH (M = No, Co) redox couples since both NiCo2S4 and NiCo2O4 are available to the electrolytes, which result in high pseudocapacitive performances. This is reflected in the comparatively larger current responses shown as well as the larger integral areas of the CV curves. The comparative CV curves at a scan rate of 5 mV s−1 (Fig. S9†) clearly show higher pseudocapacitance capability for the NCO–NCS hybrid nanostructures compared to NCO. The possible redox reactions for NiCo2S4 can be represented as follows:
| NiCo2S4 + OH− ↔ NiS4−2XOH + 2CoSXOH + 2e− | (8) |
| CoSXOH + OH− ↔ CoSXO + H2O + e− | (9) |
The shifting of the oxidation and reduction peaks towards higher and lower potentials, respectively, with an increase in scan rate indicates good electrochemical reversibility and enlarged potential separation.51 Fig. 7b and d represent the galvanostatic charge–discharge voltage curves for the NCO and NCO–NCS hybrid nanostructures at different current densities. The non-linear and asymmetric nature of the curves further confirms the pseudocapacitive nature of the electrodes. The small voltage (IR) drop in the galvanostatic discharge curves indicates the high conductivity of NiCo2O4 and NiCo2S4, which results in low equivalent series resistances (ESR) and thereby achieves a high pseudocapacitive performance.52 The specific capacitance values were calculated using the following equation:
| C = (I × Δt)/(m × ΔV) | (10) |
 | (11) |
 | (12) |
In order to investigate the electrochemical behavior of the electrodes, electrochemical impedance spectroscopy was performed for both NCO as well as the NCO–NCS samples. The Nyquist plots for both samples before and after 2000 cycles of stability test at a current density 10 A g−1 are represented in Fig. 9. In both cases the intercepts cross the real axis at the same value in the high frequency region, which indicates nearly equal values of bulk solution resistance (Rs). For NCO–NCS, a smaller semicircle than that of NCO before the cycles indicates lower interfacial charge transfer resistance (Rct). Moreover, NCO–NCS shows a more vertical line than NCO at the lower frequency region, thus indicating a lower diffusive resistance for OH− ions for NCO–NCS before the cycles. An increase in the semicircle diameter can be observed after 2000 cycles in both materials. However, NCO–NCS shows better electrochemical behavior and is found to be superior over the NCO nanosheets, which is in agreement with their stability test. As per the above electrochemical measurements, the NiCo2O4/NiCo2S4 hybrid nanostructure acts as a superior electrode material, as evident from its excellent supercapacitive performance compared to other reported ternary nanostructured materials (Table S1†). Such a high supercapacitive behavior is attributed to the synergistic effect of NiCo2O4 and NiCo2S4 as well as the morphological design of the hybrid nanostructure, as further confirmed by comparing the physically mixed NiCo2O4 and NiCo2S4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) (Fig. S13†).
1 ratio) (Fig. S13†).
 | ||
| Fig. 9 Nyquist plots of the NiCo2O4 and NiCo2O4/NiCo2S4 based electrodes before (a) and after (b) 2000 cycles (inset: corresponding magnified view at the high frequency region). | ||
The 3D architecture combining NiCo2O4 nanosheets and NiCo2S4 nanoflake balls on Ni foam exhibits an open structure, which is advantageous for alkaline electrolyte to access both the NiCo2O4 and NiCo2S4 materials and easy diffusion of OH− ions. The porous nanosheet structure not only exhibits a large number of active sites as prerequisite for redox reactions, but also facilitates directional electronic transportation and fast surface ionic diffusion which lead to fast electrochemical redox reactions. The interconnected structure of the nanosheets further supports mechanical integrity against volume expansion during several thousand cycles of charging–discharging processes.21 The direct growth of mixed metal oxides onto Ni-foam facilitates electron collection to the Ni-foam as a current collector and the strong adherence of the metal oxides offers stress-free flexibility to the electrode, as observed from the rolling-experiment shown in Fig. S14.† It can be seen that upon rolling of the electrode the specific capacitance increases slightly at a current density of 1 A g−1. This could be due to the accumulation of charges at the electrode–electrolyte interface which enhances the charge density and capacitive nature of the electrodes. Guided by this observation, the charge–discharge analysis of a doubled electrode was also performed and a further slight enhancement in the specific capacitance value can be noticed.
4. Conclusions
In conclusion, electrodes based on NiCo2O4 nanosheets and NiCo2O4/NiCo2S4 hybrid nanostructures on Ni-foam were successfully fabricated by a facile pH-controlled ammonia evaporation technique. The interconnected NiCo2O4 nanosheets are organized in a flower-like pattern, which gives rise to a three-dimensional open structure under optimum reaction conditions. NiCo2S4 nanoflake balls were anchored on to the NiCo2O4 nanosheets as a hybrid nanostructure via the same technique in the presence of thiourea as an S-precursor. This hybrid nanostructure exhibits a remarkable supercapacitive performance and allows easy access to alkaline electrolyte due to its open structure, high surface area, fast electrochemical redox reactions, fast ionic and electronic transportation and mechanical integrity due to the interconnected structure. The NiCo2O4/NiCo2S4 hybrid nanostructure manifests high specific capacitance values (3671 F g−1 at a current density of 1.8 A g−1 and 2767 F g−1 at 9 A g−1) with a capacity retention value of 84% at 10 A g−1 after 2000 cycles. The NiCo2O4/NiCo2S4 hybrid nanostructure delivers a noticeably high energy density as well as power density (8820 W kg−1 at 41.65 W h kg−1) compared to the NiCo2O4 nanosheets (5374.28 W kg−1 at 20.90 W h kg−1). Further, the electrodes show great flexibility and stability over many cycles, which promote NiCo2O4/NiCo2S4 as an advanced pseudocapacitive material for supercapacitors.Acknowledgements
P. Roy gratefully acknowledges the financial support from Science & Engineering Research Board – Department of Science and Technology, New Delhi, India for this work. S. Raj and P. Roy thank Central Instrumental Facility (C.I.F), BIT Mesra, Ranchi for their cooperation in analyzing samples. Authors would also like to thank Dr Dhamodaran Santhanagopalan, Amrita Vishwa Vidyapeetham for performing some preliminary studies. P. Roy is thankful to Ayon Karmakar and Kunal Manna, IIT Kharagpur for their help in many ways.References
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef CAS PubMed.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28–E62 CrossRef CAS PubMed.
- P. Roy and S. K. Srivastava, J. Mater. Chem. A, 2015, 3, 2454 CAS.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- J. Vatamanu and D. Bedrov, J. Phys. Chem. Lett., 2015, 6, 3594–3609 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- H. Ji, X. Zhao, Z. Qiao, J. Jung, Y. Zhu, Y. Lu, L. L. Zhang, A. H. MacDonald and R. S. Ruoff, Nat. Commun., 2014, 5, 3317 Search PubMed.
- X. Xie, C. Zhang, M.-B. Wu, Y. Tao, W. Lv and Q.-H. Yang, Chem. Commun., 2013, 49, 11092–11094 RSC.
- G. Yu, L. Hu, N. Liu, H. Wang, M. Vosgueritchian, Y. Yang, Y. Cui and Z. Bao, Nano Lett., 2011, 11, 4438–4442 CrossRef CAS PubMed.
- S. Vijayakumar, S. Nagamuthu and G. Muralidharan, ACS Appl. Mater. Interfaces, 2013, 5, 2188–2196 CAS.
- Y. Zhu, C. Cao, S. Tao, W. Chu, Z. Wu and Y. Li, Sci. Rep., 2014, 4, 5787 CrossRef CAS PubMed.
- K. Liang, X. Tang and W. Hu, J. Mater. Chem., 2012, 22, 11062–11067 RSC.
- Y. Wang, Y. Lei, J. Li, L. Gu, H. Yuan and D. Xiao, ACS Appl. Mater. Interfaces, 2014, 6, 6739–6747 CAS.
- X.-h. Xia, J.-p. Tu, Y.-j. Mai, X.-l. Wang, C.-d. Gu and X.-b. Zhao, J. Mater. Chem., 2011, 21, 9319–9325 RSC.
- D. Liu, X. Wang, X. Wang, W. Tian, J. Liu, C. Zhi, D. He, Y. Bando and D. Golberg, J. Mater. Chem. A, 2013, 1, 1952–1955 CAS.
- L. Wang, H. Ji, S. Wang, L. Kong, X. Jiang and G. Yang, Nanoscale, 2013, 5, 3793–3799 RSC.
- C. Z. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488–1504 CrossRef CAS PubMed.
- D. P. Dubal, P. Gomez-Romero, B. R. Sankapal and R. Holze, Nano Energy, 2015, 11, 377–399 CrossRef CAS.
- X. Liu, S. Shi, Q. Xiong, L. Li, Y. Zhang, H. Tang, C. Gu, X. Wang and J. Tu, ACS Appl. Mater. Interfaces, 2013, 5, 8790–8795 CAS.
- G. Q. Zhang, H. B. Wu, H. E. Hoster, M. B. Chan-Park and X. W. Lou, Energy Environ. Sci., 2012, 5, 9453–9456 CAS.
- J. Xiao, L. Wan, S. Yang, F. Xiao and S. Wang, Nano Lett., 2014, 14, 831–838 CrossRef CAS PubMed.
- J. Yang, M. Ma, C. Sun, Y. Zhang, W. Huang and X. Dong, J. Mater. Chem. A, 2015, 3, 1258–1264 CAS.
- J. Cheng, Y. Lu, K. Qiu, H. Yan, J. Xu, L. Han, X. Liu, J. Luo, J. K. Kim and Y. Luo, Sci. Rep., 2015, 5, 12099 CrossRef PubMed.
- D. Cheng, Y. Yang, J. Xie, C. Fang, G. Zhang and J. Xiong, J. Mater. Chem. A, 2015, 3, 14348–14357 CAS.
- G. Zhang and X. W. D. Lou, Adv. Mater., 2013, 25, 976–979 CrossRef CAS PubMed.
- Z. B. Wu, Y. R. Zhu and X. B. Ji, J. Mater. Chem. A, 2014, 2, 14759–14772 CAS.
- C. Yuan, J. Li, L. Hou, X. Zhang, L. Shen and X. W. D. Lou, Adv. Funct. Mater., 2012, 22, 4592–4597 CrossRef CAS.
- C. Xia, P. Li, A. N. Gandi, U. Schwingenschlögl and H. N. Alshareef, Chem. Mater., 2015, 27, 6482–6485 CrossRef CAS.
- Y. Liu, J. Zhang, S. Wang, K. Wang, Z. Chen and Q. Xu, New J. Chem., 2014, 38, 4045 RSC.
- R. Zou, Z. Zhang, M. F. Yuen, J. Hu, C. S. Lee and W. Zhang, Sci. Rep., 2015, 5, 7862 CrossRef PubMed.
- W. Xiong, Y. Gao, X. Wu, X. Hu, D. Lan, Y. Chen, X. Pu, Y. Zeng, J. Su and Z. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 19416–19423 CAS.
- D. Cai, D. Wang, C. Wang, B. Liu, L. Wang, Y. Liu, Q. Li and T. Wang, Electrochim. Acta, 2015, 151, 35–41 CrossRef CAS.
- Q. Wang, X. Wang, B. Liu, G. Yu, X. Hou, D. Chen and G. Shen, J. Mater. Chem. A, 2013, 1, 2468 CAS.
- J. Hu, M. Li, F. Lv, M. Yang, P. Tao, Y. Tang, H. Liu and Z. Lu, J. Power Sources, 2015, 294, 120–127 CrossRef CAS.
- R. K. Gupta, J. Candler, S. Palchoudhury, K. Ramasamy and B. K. Gupta, Sci. Rep., 2015, 5, 15265 CrossRef CAS PubMed.
- Q. Zhou, J. Xing, Y. Gao, X. Lv, Y. He, Z. Guo and Y. Li, ACS Appl. Mater. Interfaces, 2014, 6, 11394–11402 CAS.
- J. Wu, R. Mi, S. Li, P. Guo, J. Mei, H. Liu, W.-M. Lau and L.-M. Liu, RSC Adv., 2015, 5, 25304–25311 RSC.
- L. Shen, L. Yu, X. Y. Yu, X. Zhang and X. W. Lou, Angew. Chem., Int. Ed., 2015, 54, 1868–1872 CrossRef CAS PubMed.
- C. Zhang, T. Kuila, N. H. Kim, S. H. Lee and J. H. Lee, Carbon, 2015, 89, 328–339 CrossRef CAS.
- Q. Wang, B. Liu, X. Wang, S. Ran, L. Wang, D. Chen and G. Shen, J. Mater. Chem., 2012, 22, 21647–21653 RSC.
- L. Shen, L. Yu, H. B. Wu, X.-Y. Yu, X. Zhang and X. W. D. Lou, Nat. Commun., 2015, 6, 6694 CrossRef CAS PubMed.
- J.-G. Wang, R. Zhou, D. Jin, K. Xie and B. Wei, Energy Storage Materials, 2016, 2, 1–7 CrossRef.
- G. Q. Zhang, H. B. Wu, H. E. Hoster, M. B. Chan-Park and X. W. D. Lou, Energy Environ. Sci., 2012, 5, 9453–9456 CAS.
- Y. Li, B. Tan and Y. Wu, Chem. Mater., 2008, 20, 567–576 CrossRef CAS.
- Y. Li, B. Tan and Y. Wu, J. Am. Chem. Soc., 2006, 128, 14258–14259 CrossRef CAS PubMed.
- J. Li, S. Xiong, Y. Liu, Z. Ju and Y. Qian, ACS Appl. Mater. Interfaces, 2013, 5, 981–988 CAS.
- D. Li, Y. Gong, Y. Zhang, C. Luo, W. Li, Q. Fu and C. Pan, Sci. Rep., 2015, 3, 12903 CrossRef PubMed.
- H. Wu, G. Wu, Y. Ren, L. Yang, L. Wang and X. Li, J. Mater. Chem. C, 2015, 3, 7677–7690 RSC.
- R. Zou, M. F. Yuen, L. Yu, J. Hu, C.-S. Lee and W. Zhang, Sci. Rep., 2016, 6, 20264 CrossRef CAS PubMed.
- C. Xia and H. N. Alshareef, Chem. Mater., 2015, 27, 4661–4668 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra19376e |
| This journal is © The Royal Society of Chemistry 2016 |