Absolute configuration of the antimalarial erythro-mefloquine – vibrational circular dichroism and X-ray diffraction studies of mefloquine and its thiourea derivative†
M. Kohout*a,
J. Vandenbusscheb,
A. Rollerc,
J. Tůmaa,
J. Bogaertsd,
P. Bultinckb,
W. Herrebout*d and
W. Lindnere
aDepartment of Organic Chemistry, University of Chemistry and Technology Prague, Technická 5, 166 28 Prague, Czech Republic. E-mail: michal.kohout@vscht.cz
bDepartment of Inorganic and Physical Chemistry, Ghent University, Krijgslaan 281 (S3), 9000 Ghent, Belgium
cInstitute of Inorganic Chemistry, University of Vienna, Währinger Strasse 42, 1090 Vienna, Austria
dDepartment of Chemistry, University of Antwerp, Groenenborgerlaan 171 (G.V.023), 2020 Antwerp, Belgium. E-mail: wouter.herrebout@uantwerpen.be
eInstitute of Analytical Chemistry, University of Vienna, Währinger Strasse 38, 1090 Vienna, Austria
First published on 22nd August 2016
Abstract
The long-standing discussion of the absolute configuration of erythro-mefloquine is revisited, showcasing the strength of a combination of experimental and calculated vibrational circular dichroism spectroscopy. The resulting assignment of one of the most important antimalarials is subsequently confirmed by electronic circular dichroism spectroscopy and X-ray diffraction-based structure elucidation of thiourea derivatives, adding to the amount of evidence on the absolute configuration that had long remained a matter of debate.
Malaria remains one of the most devastating diseases in the world, responsible yearly for many hundreds of thousands of deaths as a consequence of nearly 200 million infections.1 Moreover, about 40% of the world's population lives in areas where malaria is transmitted with several hundreds of millions who live in non-endemic areas visiting endemic areas.2 Most of the 500
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths in 2013 concern children living in Africa where a child dies every minute from malaria. Although some elementary precautions help to reduce the risk, antimalarial medicines can also be used to prevent malaria or as treatment of those already infected. Moreover, the recent outbreak of Ebola in Africa strengthens the pressing necessity of malaria prevention as both deadly diseases share the same initial symptoms. Establishing that a person is a victim of Ebola requires ruling out malaria.3
000 deaths in 2013 concern children living in Africa where a child dies every minute from malaria. Although some elementary precautions help to reduce the risk, antimalarial medicines can also be used to prevent malaria or as treatment of those already infected. Moreover, the recent outbreak of Ebola in Africa strengthens the pressing necessity of malaria prevention as both deadly diseases share the same initial symptoms. Establishing that a person is a victim of Ebola requires ruling out malaria.3
As no effective vaccines are still available, antimalarial drugs are the most important tools for combatting clinical illness and for preventing disease transmission.2 One of the most effective and affordable drugs used in both prevention and as standard medication in malaria chemoprophylaxis and treatment is rac-erythro-mefloquine hydrochloride, commercialized as Lariam®.4 This molecule (see Fig. 1) is known to be chiral and both (+)-erythro-mefloquine and (−)-erythro-mefloquine have been investigated. Their stereochemistry not only influences the antimalarial activity5,6 but also results in different side effects for the respective enantiomers.7,8 While (+)-erythro-mefloquine was found to be more active against various malaria species,9 (−)-erythro-mefloquine shows longer plasma half-life than the (+)-enantiomer as well as higher affinity to adenosine receptors leading to various neuropsychiatric adverse effects.7 Despite the above mentioned impact of malaria on the lives of billions, the ensuing necessity for proper treatment medication and the detailed information on differences in activity and side effects of (+)-and (−)-erythro-mefloquine,9,10 the assignment of the absolute configuration (AC) has been quite problematic for a long time. The first assignment was based on electronic circular dichroism (ECD) data, and the authors tentatively assigned the AC of the (+)-enantiomer as (11R,12S).11 This assignment was later disproved by anomalous X-ray diffraction (XRD) of (−)-mefloquine hydrochloride for which the AC (11R,12S) was derived and hence infers the (+)-enantiomer has the (11S,12R) configuration.12 It should be noted that Karle et al.13 used X-ray diffraction (XRD) to establish the AC already in 1991. Later, do Prado et al.14 and Pitaluga et al.15 used a combination of X-ray powder diffraction and infrared spectroscopy to unambiguously determine the crystal structure of the molecule. The resulting stereochemistry is in line with that from two different approaches using residual dipolar coupling enhanced NMR spectroscopy in combination with optical rotational dispersion,16 and by XRD analysis of Mosher amides.17 The experimental data reported in ref. 9 and 10 in turn contradict data derived from stereoselective total syntheses that, until very recently, confirmed the results of the ECD measurement.18–21 The most recent stereoselective approaches based on Pd-catalyzed enantioselective borylative alkene isomerization, domino-Sonogashira-6π-electrocyclization and Heck coupling followed by Sharpless dihydroxylation not only provided stereoselectively all four stereoisomers of mefloquine but also established the AC of (+)-erythro-mefloquine as (11S,12R) and (−)-erythro-mefloquine as (11R,12S).22–24 Zhou et al.25 confirmed recently via a unique stereospecific system approach the correctness of the AC assigned by physic-chemical methods. His comment about the “unbelievable erroneous assignment by all previous five asymmetric syntheses” only underline the difficulties which can arise in such cases. To follow the concept of complementary methodologies to assign the AC of complex molecules is indeed of high scientific level.
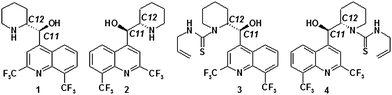 | ||
| Fig. 1 Structures of (+)-erythro-mefloquine (1), (−)-erythro-mefloquine (2), (−)-erythro-N-(N-allylthiocarbamoyl)mefloquine (3) and (+)-erythro-N-(N-allylthiocarbamoyl)mefloquine (4). | ||
Again in 2013, the AC was investigated using ECD,26 establishing the AC of the two enantiomers as well as their biological activity, thus confirming the importance of the stereochemistry in the design of mefloquine analogues that have fewer adverse effects. Clearly, such ambiguity in determining the AC of a crystalline organic compound by that many physical as well as asymmetric synthetic methods is puzzling, in particular, for an important and widely used drug. Although the debate now seems to have been settled, we here set out to establish the AC of both erythro-enantiomers using Vibrational Circular Dichroism (VCD) to show, for the first time, the unique strength of this method for establishing the AC of the present molecules. To independently confirm the VCD based results, heavy atom labeled mefloquine derivatives were synthesized (see ESI†). The applied thiourea label allows for effective crystallization and AC determination using XRD.
As the previously repeatedly used ECD method, VCD is a chiroptical spectroscopy, relying on the difference in absorption between left and right handed circular polarized infrared (IR) radiation. As a consequence, VCD offers the structural specificity of solution-based IR spectroscopy combined with stereochemical sensitivity. In general, a VCD analysis requires (1) the measurement of both IR and VCD spectra of a sample in solution with unknown AC and (2) a quantum chemical simulation of these spectra for a specific AC. Due to the differential nature of VCD spectra, both positive and negative bands can occur. This means that for a specific wavenumber, if the (+) enantiomer preferentially absorbs the right circularly polarized light, the (−) enantiomer preferentially absorbs the left circularly polarized light. The resulting spectra, however, are not so easily linked to a specific AC, hence the need for quantum chemically computed spectra. These can be computed for any AC and by carefully establishing the agreement between every computed spectrum with an experimental one, the AC of the experimental sample(s) is obtained.27,28 As reported above, ECD has been used previously but did not allow to conclusively establish the AC. One of the main advantages of VCD as opposed to ECD is that vibrational transitions are studied of which there are many more than the electronic transitions that underlie ECD.27,28 This therefore results in a more information rich set of data, reducing the risk of erroneous AC assignments. Moreover, the calculation of VCD spectra to compare experimental data is computationally less demanding than the calculation of ECD spectra, again showing an important advantage of the VCD method.29
It is important to note that mefloquine and its thiourea-derivative each contain 2 chiral centers, and hence, for a complete VCD analysis, this would require the simulation of the VCD spectra of all diastereomers. Obviously, the elucidation of the molecular AC of the sample can then only be achieved when the computed VCD spectra of the diastereomers are sufficiently different. Fortunately, however, it is known that mefloquine in Lariam has the erythro-configuration and,22 thus, the AC either corresponds to (11S,12R) or (11R,12S). One of the issues that needs to be dealt with when modelling VCD spectra is that the resulting spectra depend significantly on the molecular conformation. As the present molecules are expected to have a fairly complicated potential energy surface (PES), it is of prime importance that all significant molecular geometries are properly accounted for. This is done by conformational analysis in which all important (local) minima on the PES are identified. For all these, the IR and VCD spectra are computed leading to a molecular IR and VCD spectrum as an enthalpy based Boltzmann average over all conformations and their spectra. As exploring the PES with ab initio methods from the start is computationally too demanding, a first conformational analysis is performed at the molecular mechanics level. Three different software packages were used with the molecular mechanics force fields given between brackets: HyperChem30 (mm+31), Conflex32–35 (MMFF94S36) and Spartan37 (MMFF94 (ref. 38) and SYBYL39) each with their specific algorithms for PES exploration. The resulting minimum energy structures were then gathered and redundancies eliminated. The remaining conformations were further optimized using the B3LYP functional and the 6-31G* basis set. Solvent–solute interactions were accounted for using a Polarizable Continuum solvent Model (PCM).40 Geometry optimizations and calculations of the IR and VCD spectral data were performed using Gaussian09.41 The Boltzmann weighted IR and VCD spectra for mefloquine and N-(N-allylthiocarbamoyl)mefloquine were obtained taking into account 42 and 23 unique conformations, respectively.
The experimentally obtained VCD spectra for (+)-erythro-mefloquine and the calculated one for the (11S,12R)-configuration are shown in Fig. 2. Bands are labeled by performing the manual assignment of the bands in coherence with the IR spectra. Even though a few significant features including, amongst others, the regions near 1075, 1150 and 1450 cm−1 are less reproduced, Fig. 2 in general shows a good agreement between the spectra. Based on the visual agreement, the AC of (+)-erythro-mefloquine can clearly be assigned as (11S,12R).
The differences between the experiment and theory in Fig. 2 are believed to be due to the appearance of a strong intramolecular O–H⋯N hydrogen bond influencing both the vibrational frequencies and the IR and VCD intensities of the modes involved. To rationalize the effect of this interaction, additional calculations were performed using larger basis sets including aug-cc-pVTZ, using the B3PW91 functional, and using Grimme D3 empirical corrections for dispersion.43 Correcting the B3LYP/6-31G* data for dispersion interactions slightly strengthens the intramolecular hydrogen bond, the differences in N⋯H interatomic distances being in the order of 0.03 Å, and red shifts the modes of interest by 15 to 20 cm−1. Although the use of dispersion corrections significantly improves the agreement between the experiment and theory in the region near 1150 cm−1, it negatively affects the general agreement and the numerical descriptors used to quantify the level of agreement. The calculations using the larger basis sets and/or the B3PW91 functional confirmed the assignment reported above, but did not further improve agreement between the experiment and theory.
To avoid personal bias in manual assignments, it is recommended to establish the agreement between experimental and theoretical spectra using numerical methods. To this end we used the statistical validation approach by Vandenbussche et al.,42 where the reader is referred to this work for full technical details.
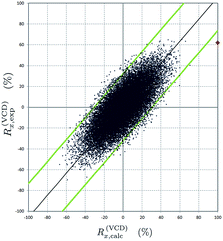
Fig. 3 shows the resulting plot which is to be interpreted as follows. A very large number of spectra are computationally generated although only one (the red dot in Fig. 3) originates from a quantum chemical and therefore physically meaningful calculation. The others (the blue dots) are quasi random spectra. In the ordinate the similarity between the computational spectra and the quantum chemical one is presented, where 100% means perfect similarity, −100% means perfect similarity with the quantum chemical spectrum of the enantiomer and 0% means complete dissimilarity. On the abscissa, the similarity between each computational spectrum and the experimental spectrum is represented. The key to a good assignment is that the quantum chemical spectrum (the red dot) must have a high similarity to the experiment (here 61%) and the blue ones must have a poorer similarity to the experimental spectrum and appear as an elongated point cloud around the bisector.
More specifically, the poorer the similarity with the quantum chemical spectrum, the poorer the agreement with the experiment. This is clearly the case here. The cloud of blue dots stretches as a fairly narrow elongated cloud along the bisector. As per ref. 34, the assignment can be considered very trustworthy.
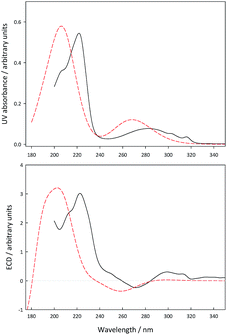
To assess the results derived above, experimental UV and ECD spectra for (+)-erythro-mefloquine were recorded in methanol and theoretical spectra for the (11S,12R)-absolute configuration were calculated at the CAM-B3LYP/PCM/6-31G* level. The spectra obtained are summarized in Fig. 4. It can be seen that in general the transitions in the UV and their corresponding features in ECD are predicted to appear at a wavelength slightly lower than those observed experimentally. The general agreement between experiment and theory, however, nicely confirms the assignment based on VCD.
The experimental and calculated spectra for 3 obtained using the B3LYP/PCM/6-31G* method are shown in Fig. 5. A manual AC assignment reveals very good agreement with the (11S,12R)-configuration, major discrepancies between experiment and theory being almost exclusively related to differences in relative intensity. The numerical, unbiased analysis (see ESI†) supports the claim that the analyzed compound has the (11S,12R)-configuration. Only few random VCD spectra are able to outperform our quantum chemical spectrum at reproducing the experimental one. That some spectra can be slightly better is to be expected for a normal distribution and given the fact that no quantum chemical calculation can be expected to fully and exactly reproduce an experiment due to inherent deficiencies in describing all intricate aspects of nature. The plot therefore reflects that assigning compound 3 to (11S,12R) is very robust, which is consistent with results obtained with XRD.
 | ||
| Fig. 5 Experimental VCD spectrum for thiourea derivative 3 (lower panel) and the computed spectrum for the (11S,12R)-absolute configuration (upper panel). | ||
Even though the AC of erythro-mefloquine seems to have converged14,16,22–26 and our VCD results support these, given the fact that previous works repeatedly contradicted each other concerning the AC of (+)-erythro-mefloquine we decided to again independently repeat single crystal XRD studies using a specifically prepared set of heavier atom containing molecules. Our above VCD based assignment agrees with previous XRD work12,14 but the reliability of the XRD data is improved thanks to considering molecules 3 and 4 which were prepared from (+)-mefloquine 1 and (−)-mefloquine 2, respectively. Their advantage is the presence of a heavier atom and thus better scatterer of the X-rays. Moreover, the X-ray based conformations may depend on the environment of the molecule, although this does not alter the AC. By performing own XRD studies, we could control all steps in our work.
As mentioned above (Fig. 1), the sign of specific rotation changed upon derivatization of (+)-mefloquine 1 and (−)-mefloquine 2 to 3 and 4. The AC, obtained from XRD analysis (Fig. 6), was determined as (11S,12R) by two independent parameter tests, Flack = 0.015(13), Hooft = 0.001(12) for the (−)-thiourea derivative 3 and as (11R,12S) by Flack = −0.001(4), Hooft = 0.001(4) for the (+)-thiourea derivative 4. Given the reported change in sign of the optical rotation, we may conclude that our VCD based assignment for (+)-erythro-mefloquine is correct and the AC of this important molecule is again established using two independent techniques.
 | ||
| Fig. 6 XRD structures of (−)-erythro-N-(N-allylthiocarbamoyl)mefloquine (3) (CCDC: 1480524†) with the absolute configuration assigned as (11S,12R) and (+)-erythro-N-(N-allylthiocarbamoyl)mefloquine (4) (CCDC: 1480523†) with absolute configuration (11R,12S). Acetonitrile is a crystal solvent; mirror plane is assigned as m. | ||
The determination of the absolute configuration of (+)-erythro-mefloquine and its enantiomer, as a racemic mixture constituting an important and highly effective antimalarial, has been used to showcase the added value of Vibrational Circular Dichroism (VCD) spectroscopy. The absolute configuration of both enantiomers was assigned on the basis of thorough statistical analysis of the VCD spectra and then independently confirmed by electronic circular dichroism and single crystal X-ray diffraction of heavy atom labeled derivates.
Notes and references
- World Health Organization, Malaria fact sheet 94, 2014.
- B. Grabias and S. Kumar, Expert Opin. Drug Saf., 2016, 15, 903–910 CrossRef CAS PubMed.
- W. Emmie de, F. Darryl, O. Clayton, R. Kyle, M. Andrea, O. Melvin, J. Bonventure, J. F. Robert, B. P. Joseph, S. David, O. Victor, O. Collins, H. Thomas, G. Allison, D. Neeltje van, Z. Galina, S. Joshua, B. Trenton, M. Kristin, R. Thomas, L. E. Shannon, F. Friederike, W. Brandi, G. N. Tolbert, G. Allen, E. S. James, K. Gary, S. Ute, R. Mark, K. B. Fatorma, C. Z. Kathryn, S. Jorgen, T. Livia, S. Martin de, T. N. Stuart, F. Barry, S. Armand, F. Heinz, M. Moses and J. M. Vincent, Emerging Infect. Dis., 2016, 22, 323–326 Search PubMed.
- P. Schlagenhauf, M. Adamcova, L. Regep, M. T. Schaerer and H.-G. Rhein, Malar. J., 2010, 9, 357–372 CrossRef PubMed.
- R. D. Shepard and A. Fletcher, Int. Patent Appl., WO 9839003, 1998.
- A. Fletcher and R. Shepherd, US Pat., 6, 2003, vol. 664, p. 397.
- Y. Bergqvist, J. Alkabbani, C. Pettersson and N. H. Huynh, J. Chromatogr., Biomed. Appl., 1993, 620, 217–224 CrossRef CAS.
- A. M. J. Croft and M. J. World, Lancet, 1996, 347, 326 CrossRef CAS.
- J. M. Karle, R. Olmeda, L. Gerena and W. K. Milhous, Exp. Parasitol., 1993, 76, 345–351 CrossRef CAS PubMed.
- T. R. Sweeney, Med. Res. Rev., 1981, 1, 281–301 CrossRef CAS PubMed.
- F. I. Carroll and J. T. Blackwel, J. Med. Chem., 1974, 17, 210–219 CrossRef CAS PubMed.
- J. M. Karle and I. L. Karle, Antimicrob. Agents Chemother., 2002, 46, 1529–1534 CrossRef CAS PubMed.
- J. M. Karle and I. L. Karle, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1991, 47, 2391–2395 CrossRef.
- V. Mendes do Prado, R. Cardoso Seiceira, A. Pitaluga Jr, T. Andrade-Filho, W. Andrade Alves, A. Reily Rocha and F. Furlan Ferreira, J. Appl. Crystallogr., 2014, 47, 1380–1386 CAS.
- A. Pitaluga Jr, L. D. Prado, R. Seiceira, J. L. Wardell and S. M. S. V. Wardell, Int. J. Pharm., 2010, 398, 50–60 CrossRef PubMed.
- M. Schmidt, H. Sun, P. Rogne, G. K. E. Scriba, C. Griesinger, L. T. Kuhn and U. M. Reinscheid, J. Am. Chem. Soc., 2012, 134, 3080–3083 CrossRef CAS PubMed.
- M. Mueller, C. M. Orben, N. Schuetzenmeister, M. Schmidt, A. Leonov, U. M. Reinscheid, B. Dittrich and C. Griesinger, Angew. Chem., Int. Ed., 2013, 52, 6047–6049 CrossRef CAS PubMed.
- R. Schmid, E. A. Broger, M. Cereghetti, Y. Crameri, J. Foricher, M. Lalonde, R. K. Muller, M. Scalone, G. Schoettel and U. Zutter, Pure Appl. Chem., 1996, 68, 131–138 CrossRef CAS.
- Z.-X. Xie, L.-Z. Zhang, X.-J. Ren, S.-Y. Tang and Y. Li, Chin. J. Chem., 2008, 26, 1272–1276 CrossRef CAS.
- J. D. Knight, S. J. Sauer and D. M. Coltart, Org. Lett., 2011, 13, 3118–3121 CrossRef CAS PubMed.
- W. P. Hems, W. P. Jackson, P. Nightingale and R. Bryant, Org. Process Res. Dev., 2012, 16, 461–463 CrossRef CAS.
- J. Ding and D. G. Hall, Angew. Chem., Int. Ed., 2013, 52, 8069–8073 CrossRef CAS PubMed.
- N. Schuetzenmeister, M. Mueller, U. M. Reinscheid, C. Griesinger and A. Leonov, Chem.–Eur. J., 2013, 19, 17584–17588 CrossRef CAS PubMed.
- E. J. Rastelli and D. M. Coltart, Angew. Chem., Int. Ed., 2015, 54, 14070–14074 CrossRef CAS PubMed.
- G. Zhou, X. Liu, X. Liu, H. Nie, S. Zhang and W. Chen, Adv. Synth. Catal., 2013, 355, 3575–3580 CrossRef CAS.
- A. Dassonville-Klimpt, C. Cézard, C. Mullié, P. Agnamey, A. Jonet, S. Da Nascimento, M. Marchivie, J. Guillon and P. Sonnet, ChemPlusChem, 2013, 78, 642–646 CrossRef CAS.
- Y. He, B. Wang, R. K. Dukor and L. A. Nafie, Appl. Spectrosc., 2011, 65, 699–723 CrossRef CAS PubMed.
- S. S. Wesolowski and D. E. Pivonka, Bioorg. Med. Chem. Lett., 2013, 23, 4019–4025 CrossRef CAS PubMed.
- G. Magyarfalvi, G. Tarczay and E. Vass, Wiley Interdiscip. Rev. Comput. Mol. Sci., 2011, 1, 403–425 CrossRef CAS.
- HyperChem, Hypercube, Inc., 1115 NW 4th Street, Gainesville, Florida 32601, USA Search PubMed.
- N. L. Allinger, J. Am. Chem. Soc., 1977, 99, 8127–8134 CrossRef CAS.
- H. Goto, K. Ohta, T. Kamakura, S. Obata, N. Nakayama, T. Matsumoto and E. Osawa, Conflex, Tokyo-Yokohama, Japan, 2004 Search PubMed.
- H. Goto and E. Osawa, J. Am. Chem. Soc., 1989, 111, 8950–8951 CrossRef CAS.
- H. Goto and E. Osawa, J. Chem. Soc., Perkin Trans. 2, 1993, 187–198 RSC.
- Conflex, Conflex Corporation, 3-23-17, Takanawa, Minato-ku, Tokyo 108-0074, Japan Search PubMed.
- T. Halgren, J. Comput. Chem., 1999, 20, 720–729 CrossRef CAS.
- Spartan, Wavefunction Inc., 18401 Von Karman Avenue, Suite 370, Irvine, CA 92612, USA Search PubMed.
- T. Halgren, J. Comput. Chem., 1996, 17, 490–519 CrossRef CAS.
- M. Clark, R. D. Cramer and N. Vanopdenbosch, J. Comput. Chem., 1989, 10, 982–1012 CrossRef CAS.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3094 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian Inc., Wallingford, CT, 2009.
- J. Vandenbussche, P. Bultinck, A. K. Przybyl and W. A. Herrebout, J. Chem. Theory Comput., 2013, 9, 5504–5512 CrossRef CAS PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Chromatographic resolution of enantiomers, synthetic details, NMR spectra, crystallographic details, and the results of quantum chemistry calculations. CCDC 1480523 and 1480524. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra19367f |
| This journal is © The Royal Society of Chemistry 2016 |