DOI:
10.1039/C6RA19362E
(Paper)
RSC Adv., 2016,
6, 83288-83295
Enhancing the adsorption behavior and mechanism of Sr(II) by functionalized montmorillonite with different 3-aminopropyltriethoxysilane (APTES) ratios†
Received
31st July 2016
, Accepted 17th August 2016
First published on 17th August 2016
Abstract
Modified calcium saturated montmorillonite (Mt) was prepared at different 3-aminopropyltriethoxysilane (APTES) loadings through grafting. The as-products were characterized by XRD, FTIR and elemental analysis. Surface analysis such as acidity was examined at different H+ concentrations, showing that the release of OH− from the modified samples increased in the following order: APTES4.0CEC–Mt > APTES3.0CEC–Mt > APTES2.0CEC–Mt > APTES1.0CEC–Mt. Batch experiments were conducted and different conditions (pH, temperature and co-existing ions) were used to evaluate the adsorption capacity of the modified samples. The study developed a new way to confirm the mechanism, which indicated that ligand adsorption was the dominant sorption mechanism for Sr(II) uptake onto APTES–Mts. In general, APTES grafted montmorillonite possessed a higher efficiency of Sr(II) uptake and showed great potential for Sr(II)-rich wastewater treatment.
1. Introduction
With the development of nuclear technology and industry activities, water and soil contaminated by radio-nuclides and toxic metals pose serious environmental threats and thus challenge in remediation.1 Strontium isotopes, including Sr90 and Sr89, are an important source of highly soluble radioactivity in waste water.2 Excess strontium causes serious water and soil pollution because it is one of the most hazardous radioactive contaminants of the environment, which can move with aqueous media in the subsurface3 and even be retained in organisms such as plants, animals and human bodies.4,5 Therefore, the development of safe, simple and highly efficient methods for radioactive waste treatment is clearly required.
Many physicochemical and biologic methods, such as adsorption, chemical precipitation, ion exchange and membrane processes, have been developed for the removal of radioactive pollutants. Among these techniques, the adsorption process is considered to be effective and economical, which has been used to remove organic and metal ion matter from wastewater for many years due to its high selectivity, radiation stability, and good compatibility.6–8 These adsorption methods are always based on the use of clay for due to its highly efficient and convenient properties. In particular, montmorillonite (Mt) is widely used for the elimination of pollutants from wastewater because of its high surface area, swelling property, and high cation exchange capacity (CEC).9 However, considering its limited affinity to some radionuclides, different modification methods, particularly organic modification, have relatively improved the adsorption capacity of natural clay minerals. Hang Long investigated ethylamine functionalized montmorillonite, which had a significant improvement of the surface properties and a highly adsorption capacity for Cs+.10 Papachristodoulou reported that the material of Al-pillared montmorillonite (Al-Mt) carrying carboxylate functional groups was highly improved for Sr(II) adsorption.11 Bors discussed that MX-80 Wyoming-bentonite treated with hexadecylpyridinium (HDPy+) displayed great performance in the removal of Cs+ and Sr2+.12
3-Aminopropyltriethoxysilane (APTES) is a type of silane coupling agent with the functional group NH2, which has been recommended to modify clay minerals13–15 and provide many adsorption sites for pollutants.16 In our previous works, we reported the comparison for Sr(II) adsorption by the modified montmorillonite with sodium dodecyl sulfonate (SDS), hexadecyl trimethyl ammonium bromide (HDTMAB) and APTES. The experimental results demonstrated that APTES–Mt has the maximum adsorption capacity and strongest affinity to Sr(II) compared to SDS–Mt or HDTMAB–Mt.2 In addition, different sorption mechanisms are demonstrated, which are strongly associated with the molecular structure of pollutants and modifiers in functionalized clays.17 The adsorption behavior of organoclays prepared by different types of modifiers may create different adsorption mechanisms, which significantly affect the adsorption capacity of organoclays.18 This means that adsorptive property of materials is depended greatly on the arrangement of intercalated organic molecular within the interlayer of clay. Moreover, the co-existing ion, pH and equilibrium temperature of the solution are important factors that also could strongly influence the adsorption performance.19 Therefore, in-depth studies should be conducted for a better understanding of the relationship between silane modifiers arrangement and the adsorption behavior of silane functionalized organoclays.
In the current study, organo-montmorillonite was synthesized from 3-aminopropyltriethoxysilane with different loading rates and used for the removal of Sr(II). An idea was introduced to provide a detailed description of the mechanisms by detecting the concentration of Ca(II) and Sr(II) in a solution before and after the adsorption process. The aim of this article was to discuss the influence of the quantity of APTES to the as-products and deeply investigate the adsorption mechanism of Sr(II).
2. Experiments
2.1. Materials
The experimental reagents were of analytical grade and obtained from Guangzhou Chemical Reagent Factory, Guangdong Province, China, except APTES, which was purchased from Aladdin.
Montmorillonite used was saturated with calcium (Mt) and the basal d-spacing is 15.9 Å. This clay was supplied by Fei Lai Feng Nonmetal Mineral Material Co. Ltd, Guangdong province, China. Its chemical composition was SiO2; Al2O3; Fe2O3; MgO; CaO; Na2O; K2O.
2.2. Apparatus
A PW-4400 X-ray fluorescence spectrometer (XRF) was used to analyze the chemical composition of all the materials with a 60 kV generator and a 4 kW rhodium X-ray tube. A Bruker D8 diffractometer system with Cu-Kα radiation (λ = 1.54 Å) was used for X-ray analysis. The XRD study was performed from 2° to 10° (2θ) at a rate of 0.02° s−1. Fourier-transform infrared (FTIR) spectroscopy was performed using a FTIR spectrometer from 4000 to 400 cm−1 (American 96 Thermo-electron Corporation). pH was recorded by a PHS-3C acidimeter. The adsorption data were recorded on a Hitachi Z-2000 Polarized Zeeman Atomic Absorption Spectrophotometer (AAS).
2.3. Preparation of APTES–Mts
APTES–Mts were prepared as follows.2,13,14 2.5 g dried Mt was dispersed in 50 mL cyclohexane followed by ultrasonic dispersion to scatter the mixture adequately. A certain amount of APTES was then added to the abovementioned suspension with a ratio of nAPTES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mMt = 0.78 mmol g−1 (1CEC), 1.56 mmol g−1 (2CEC), 2.34 mmol g−1 (3CEC), 3.12 mmol g−1 (4CEC). The obtained materials were accordingly defined as APTES1.0CEC–Mt, APTES2.0CEC–Mt, APTES3.0CEC–Mt, and APTES4.0CEC–Mt. Finally, the prepared products were dried in an oven, pulverized to pass through a 200 μm mesh sieve, and sealed for the next step.
mMt = 0.78 mmol g−1 (1CEC), 1.56 mmol g−1 (2CEC), 2.34 mmol g−1 (3CEC), 3.12 mmol g−1 (4CEC). The obtained materials were accordingly defined as APTES1.0CEC–Mt, APTES2.0CEC–Mt, APTES3.0CEC–Mt, and APTES4.0CEC–Mt. Finally, the prepared products were dried in an oven, pulverized to pass through a 200 μm mesh sieve, and sealed for the next step.
2.4. Adsorption of Sr(II) and methods
A Sr(II) stock solution (240 mg L−1) was prepared by dissolving SrCl2 into distilled water. The batch adsorption behaviors of Sr(II) on to the materials were conducted through a variety of methods under different conditions. A 25 mL Sr(II) solution added to a 50 mL beaker was stirred using a magnetic stirrer, mixing with 50 mg adsorbent. Solution pH, temperature and existing ions were investigated under the conducted conditions with primary Sr(II) concentration of 240 mg L−1 and contact time of 24 h. pH was adjusted to 1.0–11.0 to investigate the impact of the pH value. The temperature condition was examined at 30 °C, 40 °C, 50 °C and 60 °C. Anions, such as nitrate, sulphate, phosphate, acetate and chlorate, were added to investigate the adsorption influence of these ions. The initial concentration of Sr(II) was between 20 and 300 mg L−1, and the adsorption time conditions were set as 5 min to 8 d.2 In addition, 0.05 mmol L−1 was fixed by adding KCl into all the abovementioned Sr(II) solutions to maintain ionic strength. To deeply analyze the adsorption mechanism of Sr(II), the concentration of Ca(II) ions was also measured before and after adsorptions. Atomic adsorption spectrometry (AAS) was used to determine the concentration of Sr(II) and Ca(II) ions for all samples. The parameters for Sr(II) analyzing include concentration range 0–32 mg L−1, wavelength 460.7 nm, lamp current 8.0 mA, slit width 1.3 nm, C2H2 flow (the fuel) 2.0 L min−1, and air flow (the oxidant) 15.0 L min−1, and that for Ca(II) analyzing include concentration range 0–8 mg L−1, wavelength 422.7 nm, lamp current 7.0 mA, slit width 1.3 nm, C2H2 flow (the fuel) 2.4 L min−1, and air flow (the oxidant) 15.0 L min−1. All the experiments were performed twice. The adsorption capacity of Sr(II) q (mg g−1) and the exchanged capacity qi (mmol g−1) were calculated as follows.| |
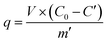 | (1) |
| |
 | (2) |
where V (mL) is the volume of solution, C0 and C′ (mg L−1) are the Sr(II) and Ca(II) concentration before and after the adsorption tests in solution, m′ is the amount of adsorbent, and M is the molar mass of Sr(II) and Ca(II).
3. Results and discussion
3.1. Structural characteristics
3.1.1. XRD and FTIR results. As shown in Fig. 1, the XRD patterns of Mt and APTES–Mts were all in the low angle region (2θ). The d001 value was 1.59 nm, 1.55 nm, 1.74 nm, 2.00 nm, and 1.99 nm for Mt, APTES1.0CEC–Mt, APTES1.5CEC–Mt, APTES2.0CEC–Mt, APTES2.5CEC–Mt, APTES3.0CEC–Mt, and APTES4.0CEC–Mt, respectively. Obviously, the d value for the Mt grafted reflection increased with increasing dosage of APTES from 1.59 nm to 2.00 nm. This observation is likely due to the arrangement of flatly lying APTES molecules in the monolayer in the interlayer space of Mt.20 With increasing APTES loading, the (001) reflection of APTES–Mt gradually becomes weaker, demonstrating the possible formation of a disordered pillared structure, resulting from the aggregation of clay platelets.21 Mt functionalized with APTES exhibited some new vibration bands (Fig. S1 in ESI†). After treatment of Mt with APTES, new bands at 2925, 2933, 2932 cm−1 appeared, the intensity of the band at 3624 cm−1 decreased slightly when the amount of APTES was increased. These changes indicate that APTES was linked to the aluminol surface of Mt.22 Other new peaks for all APTES-modified Mt at 1507 cm−1, 1509 cm−1, 1512 cm−1, and 1513 cm−1 belong to N–H symmetric flexing. The CH3 asymmetric flexing and C–H flexing at 1448 cm−1, 1450 cm−1 and 1414 cm−1, and 1419 cm−1 were not distinguished until the dosage of APTES was 4CEC. In addition, four peaks at 697, 697, 695, and 694 cm−1 were shown at all the modified samples due to O–Si–O asymmetric stretching. These observations gave some additional support for the grafting of APTES.
 |
| | Fig. 1 X-ray patterns of Mt and grafted samples. | |
3.1.2. BET and XRF analysis. The BET surface area and pore volume for and APTES–Mts decreased gradually in the order given below: APTES1.0CEC–Mt > APTES2.0CEC–Mt > APTES3.0CEC–Mt > APTES4.0CEC–Mt (Table S1†). The phenomenon of the SBET decrease one by one is possibly due to increased immobilized organic ligands grafted on the edge of the clay sheets with the amount of APTES, which blocks the entrance to some structural channels, thus leading to a less nitrogen adsorption quantity corresponding to the N2 adsorption–desorption isotherms.23 In addition, this assignment was well supported by XRF analysis (Table 1). The small decreases in the contents of K and Ca demonstrated that APTES entered into the interlayer of Mt via ion-exchange successfully. The increased percentage of Si after modification also indicated that APTES had been grafted onto Mt. However, 1CEC APTES functionalization leads to a remarkable increase in the specific surface area (SBET) and microporous volume (Vmicro) compared to Mt. These are possibly attributed to the strongly sorbed APTES and partial occupancy of the clay interlayer when the amount of APTES was low during modification.10
Table 1 Elemental analysis of Mt and APTES–Mts
| Sample |
O (%) |
Si (%) |
Al (%) |
Fe (%) |
Mg (%) |
Ca (%) |
Na (%) |
K (%) |
| Mt |
46.015 |
30.291 |
8.125 |
3.533 |
2.760 |
2.258 |
0.018 |
0.173 |
| APTES(1.0CEC)–Mt |
46.277 |
31.2 |
7.613 |
3.275 |
2.536 |
2.093 |
0.036 |
0.161 |
| APTES(2.0CEC)–Mt |
44.88 |
30.78 |
6.952 |
3.009 |
2.339 |
1.93 |
0.01 |
0.152 |
| APTES(3.0CEC)–Mt |
45.182 |
31.355 |
6.658 |
2.919 |
2.289 |
1.853 |
0.016 |
0.141 |
| APTES(4.0CEC)–Mt |
46.888 |
32.9 |
6.721 |
2.864 |
2.233 |
1.752 |
— |
0.134 |
In addition, APTES1.0CEC–Mt, APTES2.0CEC–Mt, APTES3.0CEC–Mt and APTES4.0CEC–Mt exhibited the pHzpc values of about 2.2, 6.0, 7.8 and 8.3, respectively, which are higher than that of Mt. The increase in pHzpc of APTES–Mts can be explained by ammonia tailoring of APTES grafted on the surface of Mt becoming more basic, thus created a more positively charged surface.24 These results indicated that the dosage of loaded APTES plays an important role in the distribution of the structure of APTES–Mt and it could further determine the sorption mechanisms.
3.1.3. Surface properties. The surface properties of APTES–Mts were determined by adding H+ to measure the pH value of solution. Fig. 2 shows the variation of the equilibrium pH during the addition of HCl. The results showed the same trend during the full H+ condition on five different materials. As demonstrated, different buffer regions displayed differently at the initial part, whereas they had similar profiles at a higher H+-loading. The pH was larger with a larger loading of APTES, showing that the release of OH− from the modified samples increased as APTES4.0CEC–Mt > APTES3.0CEC–Mt > APTES2.0CEC–Mt > APTES1.0CEC–Mt. From the results, the cooperation of protonation reactions and hydrolysis was important to the reactions. The quantity of APTES on Mt affected the pH of the suspension. The involved reactions are as follows:| |
 | (3) |
| | |
NH2–(CH2)3–Si(OH)3 + H2O → Si(OH)3–(CH2)3–NH2·H+ + OH−
| (4) |
 |
| | Fig. 2 Variation of pH versus the quantity of hydrogen ions added to the suspension of Mt and APTES–Mts. | |
It could be concluded that in the range of pH > 3.5, the reaction was dominated by the hydroxyl-equilibrium caused by the protonation of –NH2 groups.
3.2. Adsorption results
3.2.1. Sr(II) ions adsorption on different loading materials. The adsorption of Sr(II) on Mt and APTES–Mts was investigated under the condition that the contact time was 24 hours, the initial Sr(II) concentration was 240 mg L−1, and the temperature was chosen as room temperature. As shown in Fig. 3, the adsorption capacity greatly increased after APTES-modification with increasing CEC ratios APTES–Mt. In this experiment, the increasing CEC ratios may lead to more functional groups exposed to the outside that provide more binding sites for Sr(II). Obviously, the adsorption capacity for five materials was 12.11 mg g−1, 52.13 mg g−1, 60.5 mg g−1, 71.95 mg g−1, and 70.63 mg g−1 for Mt, APTES1.0CEC–Mt, APTES2.0CEC–Mt, APTES3.0CEC–Mt, and APTES4.0CEC–Mt, respectively. Therefore, the optimal adsorbent with 3CEC ratios of APTES to Mt, and APTES3.0CEC–Mt was chosen for further tests.
 |
| | Fig. 3 Adsorption of Sr(II) on Mt and APTES–Mts (contact time: 24 h; initial concentration 240 mg L−1; temperature 30 °C; pH 6.0; adsorbent dosage: 2.0 g L−1). | |
3.2.2. Effect of solution pH on Sr(II) adsorption. The solution pH is significant in affecting the adsorption of metal ions.25–27 The effect of pH on the Sr(II) adsorption to the raw Mt and APTES–Mts is shown in Fig. 4. It could be seen that the Sr(II) uptake by APTES–Mts was highly pH-dependent and the adsorption capacity increased with the increasing pH, whereas raw Mt presents no distinct change and maintains at below 16.25 mg g−1 at experimental pH values ranging from 1.0 to 10.0, which is in agreement with a previous report on natural clinoptilolite in the same pH range.28 APTES–Mts had higher adsorption quantity of Sr(II) than Mt on the chosen pH condition, which could be associated with the functional amino group (–NH2). At a low pH, excessive H+ competes with Sr2+ for the binding sites of –NH2, which was of alkalescence and could be protonated, even at high pH level.29 At a high pH, OH− neutralized H+ from –NH3+, and the number of negatively charged surface sites increases, thus enhance Sr(II) removal.30
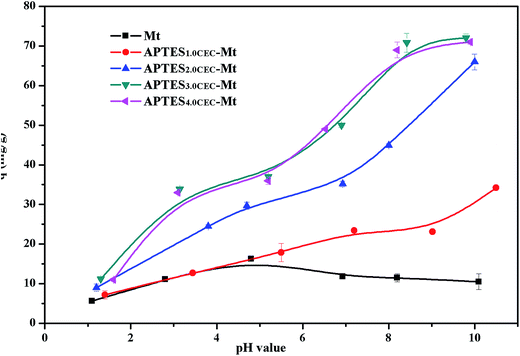 |
| | Fig. 4 Effect of pH for adsorption on Mt and APTES–Mts (contact time: 24 h; initial concentration: 240 mg L−1; temperature: 30 °C; adsorbent dosage: 2.0 g L−1). | |
3.2.3. Effect of temperature on Sr(II) adsorption. Fig. 5 shows that the adsorption quantity increased slightly with increasing temperature. To determine the influence of temperature on adsorption, thermodynamic parameters, such as ΔG, ΔH and ΔS, were estimated using the following equations:31| |
 | (5) |
| |
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kd kd
| (6) |
| |
 | (8) |
where kd(qe/Ce) (mL g−1) is the distribution coefficient, R is the universal gas constant (8.314 J mol−1 K−1) and T is temperature (K). qe (mg g−1) and Ce (mg L−1) are the adsorbing capacity and the concentration of Sr(II) on the adsorbent at equilibrium, respectively; the thermodynamic parameters, such as ΔG, ΔH and ΔS, indicate the changes in the Gibbs free energy, the enthalpy and entropy, respectively, ΔH and ΔS are decided by eqn (8) with the slope and intercept of Van't Hoff plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kd versus 1/T; R (8.314 J mol−1 K−1) is the gas law constant and T (K) is the absolute temperature. The thermodynamic parameters calculated are listed in Table 2. The positive values of ΔG for Mt, APTES1.0CEC–Mt and APTES2.0CEC–Mt indicate the non-spontaneous adsorption process, whereas the negative values for APTES3.0CEC–Mt and APTES4.0CEC–Mt indicate the spontaneous process. ΔH was positive for all materials, which means the adsorption processes are endothermic. Furthermore, the positive values of ΔS describe the increasing randomness in the solid/solution interface during the adsorption process.32
kd versus 1/T; R (8.314 J mol−1 K−1) is the gas law constant and T (K) is the absolute temperature. The thermodynamic parameters calculated are listed in Table 2. The positive values of ΔG for Mt, APTES1.0CEC–Mt and APTES2.0CEC–Mt indicate the non-spontaneous adsorption process, whereas the negative values for APTES3.0CEC–Mt and APTES4.0CEC–Mt indicate the spontaneous process. ΔH was positive for all materials, which means the adsorption processes are endothermic. Furthermore, the positive values of ΔS describe the increasing randomness in the solid/solution interface during the adsorption process.32
 |
| | Fig. 5 Effect of temperature and absorption thermodynamics for Sr(II) removal on Mt and APTES–Mts (contact time: 24 h; initial concentration: 240 mg L−1; pH 6.0; adsorbent dosage: 2.0 g L−1). | |
Table 2 Thermodynamic parameters for Sr(II) removal by Mt and APTES–Mts
| Sample |
−ΔH (kJ mol−1) |
ΔS (J mol−1 K−1) |
−ΔG (kJ mol−1) |
| 30 °C (303 K) |
40 °C (313 K) |
50 °C (323 K) |
60 °C (333 K) |
| Mt |
−12.380 |
22.382 |
−5.630 |
−5.410 |
−5.024 |
−5.005 |
| APTES1.0CEC–Mt |
−9.295 |
26.444 |
−1.257 |
−1.137 |
−0.628 |
−0.537 |
| APTES2.0CEC–Mt |
−5.687 |
16.986 |
−0.561 |
−0.348 |
−0.199 |
−0.042 |
| APTES3.0CEC–Mt |
−6.510 |
24.470 |
0.909 |
1.111 |
1.429 |
1.625 |
| APTES4.0CEC–Mt |
−4.556 |
17.341 |
0.715 |
0.815 |
1.087 |
1.207 |
3.2.4. Effect of coexisting anions on Sr(II) adsorption. Fig. 6 shows the effect of coexisting anions such as nitrate, sulphate, phosphate, acetate and chlorate to the Sr(II) adsorption onto APTES3.0CEC–Mt under a constant condition for each anion. As shown, the effect on the Sr(II) adsorption capacity of those anions followed the order: SO42− > Cl− > NO3− ≈ CH3COO− > H2PO4−. This reflected the different affinities of these anions to the active surface sites of adsorbents. Obviously, SO42− nearly did not affect the adsorption process, whereas a slight decrease was demonstrated for the adsorption capacity when Cl− existed. The coexisting NO3− and CH3COO− increased the inhibition of Sr(II) adsorption. However, the Sr(II) uptake in the presence of H2PO4− was strongly retarded with a reduction of almost 20 mg g−1. The reason for the influence of existing ions to Sr(II) adsorption could be contributed to the competition for the active surface sites.5
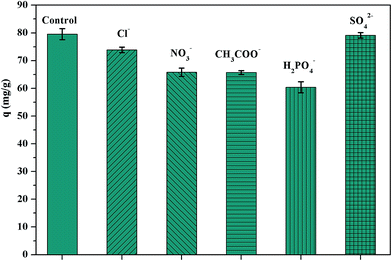 |
| | Fig. 6 Effect of coexisting anions on Sr(II) adsorption by APTES3.0CEC–Mt (contact time: 24 hours; Sr(II) concentration: 240 mg L−1; temperature: 30 °C; pH: 6.0; adsorbent dose: 2 g L−1). | |
3.2.5. Analysis of adsorption mechanism. As shown in Fig. 7, the contents of Ca(II) and Sr(II) dissolved by adsorbents in distilled water and 240 mg L−1 Sr(II) solution were investigated for the further step into the adsorption mechanism. For raw Mt, the amount of Ca(II) (mmol g−1) exchanged in the water solution (A1) was the same at that exchanged in the Sr(II) solution (A2) and Sr(II) adsorbed on Mt (A3) was equal to that of A1 and A2. This proved that ion exchange was predominant in the process of Sr(II) adsorption on Mt, as shown in Fig. 8(a). Moreover, Sr(II) can also be linked to hydroxyl groups of phyllosilicates sheet, as shown in Fig. 8(b).33 For APTES–Mts, the Ca(II) exchanged in a Sr(II) solution (A2) was more than that of Mt (A1), and the Sr(II) adsorbed on materials (A3) was larger than A2. This demonstrated that another adsorption mechanism appeared for APTES–Mts and it was more important than ion-exchange, proven from the adsorption quantity analyzed above. Based on the structural peculiarity, Fig. 7 showed that the value of A3 was much larger than A2 and the adsorption could be related to the coordination of –NH2 and Si–O, which were important ligands, as shown in Fig. 8(c) and (d). In addition, the value of A3 was much larger than A2, it could be concluded that coordination–adsorption might be dominant for Sr(II) adsorption on APTES–Mts. The mechanism of Sr(II) adsorption on APTE grafted clay sample can be summarized using the following equations.10| |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) XCa + Sr2+ ↔ XCa + Sr2+ ↔ ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) XSr + Ca2+ XSr + Ca2+
| (9) |
| |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SOH + Sr2+ ↔ SOH + Sr2+ ↔ ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SOSr + H+ SOSr + H+
| (10) |
| |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) R–NH2 + Sr2+ ↔ R–NH2 + Sr2+ ↔ ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) R–NH2Sr2+ R–NH2Sr2+
| (11) |
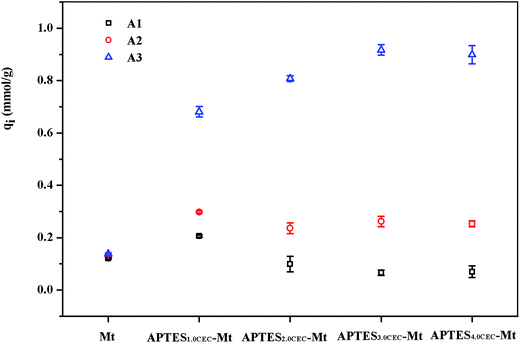 |
| | Fig. 7 Adsorption mechanisms analysis (A1 stands for Ca(II) content in water solution as control test, A2 stands for Ca(II) content in Sr(II) solution, A3 stands for Sr(II) content adsorbed on materials). | |
 |
| | Fig. 8 Hypothetical simulation of Sr2+ adsorption on APTE–Mts. | |
4. Conclusions
Different amounts of APTES grafted montmorillonites were synthesized and used to study adsorption mechanism of Sr(II). The surface proton concentration increased with increasing APTES-modification. The XRD patterns and FTIR spectra illustrated that the APTES had been intercalated into the interlayer of raw Mt and the d001 increased from 1.0CEC modification to 3.0CEC modification, whereas a slight decrease for 4.0CEC modification demonstrated that excess APTES would block the pores of the surface.
The adsorption tests proved that the Sr(II) uptake by APTES3.0CEC–Mt was the best with 73 mg g−1. This indicated that the amount of APTES would influence the adsorption ability of APTES–Mts with an increase until 3.0CEC. The solution pH would affect the adsorption process and improve the adsorption capacity under a high optimal range. The thermodynamic parameters demonstrated that the Sr(II) removal process on APTES–Mts was non-spontaneous and endothermic in nature. The coexisting anions, such as H2PO4−, CH3COO− and NO3−, were retarded markedly the Sr(II) adsorption, and Cl− followed, whereas SO42− exhibited barely significant effect. Adsorption mechanisms analysis showed that the coordination–adsorption was dominant for Sr(II) adsorption on APTES–Mts.
These results indicated that APTES3.0CEC–Mt could restore the Sr(II)-rich wastewater effectively.
Acknowledgements
The authors are thankful to the financial support by the National Science Foundation of China (Grant No. 41673092, 41472038, 41273122, 41073058), the Science and Technology Plan of Guangdong Province, China (No. 2014A020216002), Science and Technology Program of Guangzhou, China (No. 201604020064) and the Fundamental Research Funds for the Central Universities, SCUT (No. 2015ZP007).
References
- B. C. Bostick, M. A. Vairavamurthy, K. G. Karthikeyan and J. Chorover, Environ. Sci. Technol., 2002, 36, 2670–2676 CrossRef CAS PubMed.
- W. Pingxiao, D. Yaping, L. Hang, Z. Nengwu, L. Ping, W. Jinhua and D. Zhi, Chem. Eng. J., 2012, 191, 288–296 CrossRef.
- S. Lin, J. Hazard. Mater., 2002, 92(3), 315–326 CrossRef CAS PubMed.
- N. M. Kocherginsky, Y. K. Zhang and J. W. Stucki, Desalination, 2002, 144(1), 267–272 CrossRef CAS.
- Z. Jianbing, W. Pingxiao, D. Zhi, Z. Nengwu, L. Ping, W. Jinhua and W. Xiangde, Chem. Eng. J., 2010, 162(3), 1035–1044 CrossRef.
- A. Mellah, A. Silem, A. Boualia and R. Kada, Chem. Eng. Process., 1992, 31, 191–194 CrossRef CAS.
- M. Abdelhamid, C. Salah and S. Louisa, Int. J. Miner. Process., 1994, 41, 295–303 CrossRef.
- L. Zhang, J. Wei, X. Zhao, F. Li, F. Jiang, M. Zhang and X. Cheng, Chem. Eng. J., 2016, 285, 679–689 CrossRef CAS.
- M. E. Ramos and F. J. Huertas, Appl. Clay Sci., 2014, 90, 27–34 CrossRef CAS.
- H. Long, P. Wu and N. Zhu, Chem. Eng. J., 2013, 225, 237–244 CrossRef CAS.
- C. Papachristodoulou, J. Colloid Interface Sci., 2002, 245(1), 32–39 CrossRef CAS PubMed.
- E. Başçetin and G. Atun, Appl. Radiat. Isot., 2006, 64(8), 957–964 CrossRef PubMed.
- H. He, J. Duchet, J. Galy and J.-F. Gerard, J. Colloid Interface Sci., 2005, 288(1), 171–176 CrossRef CAS PubMed.
- S. Wei, H. Hongping, Z. Jianxi, Y. Peng and L. F. Ray, J. Colloid Interface Sci., 2007, 313(1), 268–273 CrossRef PubMed.
- Q. Zonghua, Y. Peng, Z. Jianxi, H. Hongping, L. Dong and Y. Shuqin, Appl. Clay Sci., 2010, 50(4), 546–553 CrossRef.
- S. Kang, Clays Clay Miner., 2001, 49, 119–125 Search PubMed.
- A. S. James and G. Adina, Environ. Sci. Technol., 1995, 29(3), 685–692 CrossRef PubMed.
- D. R. Paul, Q. H. Zeng, A. B. Yu and G. Q. Lu, J. Colloid Interface Sci., 2005, 292(2), 462–470 CrossRef CAS PubMed.
- Z. Ying, S. Zhenyi, C. Changlun, H. Jun and C. Hongli, Appl. Clay Sci., 2014, 87, 1–6 CrossRef.
- S.-q. Yang, P. Yuan, H.-p. He, Z.-h. Qin, Q. Zhou, J.-x. Zhu and D. Liu, Appl. Clay Sci., 2012, 62–63, 8–14 CrossRef CAS.
- G. Lagaly and S. Ziesmer, Adv. Colloid Interface Sci., 2003, 100, 105–128 CrossRef.
- J. Matusik, A. Gaweł and K. Bahranowski, Appl. Clay Sci., 2012, 56, 63–67 CrossRef CAS.
- L. Tran, P. Wu, Y. Zhu, S. Liu and N. Zhu, Appl. Surf. Sci., 2015, 356, 91–101 CrossRef CAS.
- X. Peng, F. Hu, F. L. Y. Lam, Y. Wang, Z. Liu and H. Dai, J. Colloid Interface Sci., 2015, 460, 349–360 CrossRef CAS PubMed.
- l. C. Özero and G. Keçeli, J. Radioanal. Nucl. Chem., 2006, 268, 211–219 CrossRef.
- Z. Long, M. Hiroshi, Y. Fumio and K. Tamikazu, J. Appl. Polym. Sci., 2004, 91, 556–562 CrossRef.
- W. Min, X. Ling, Z. Maolin, P. Jing, L. Jiuqiang and W. Genshuan, Carbohydr. Polym., 2008, 74, 498–503 CrossRef.
- I. Smičiklas, S. Dimović and I. Plećaš, Appl. Clay Sci., 2006, 35, 139–144 CrossRef.
- P. Luo, B. Zhang, J. H. Wang, Y. F. Zhao, J. D. Liu and J. S. Zhang, Ind. Eng. Chem. Res., 2011, 50, 10246–10252 CrossRef CAS.
- S.-Z. Li and P.-X. Wu, J. Hazard. Mater., 2010, 173, 62–70 CrossRef CAS PubMed.
- A. K. Bhattacharya, T. K. Naiya, S. N. Mandal and S. K. Das, Chem. Eng. J., 2007, 137, 529–541 Search PubMed.
- Q. Li, L. Chai, Z. Yang and Q. Wang, Appl. Surf. Sci., 2009, 255, 4298–4303 CrossRef CAS.
- T.-H. Wang, C.-J. Hsieh, S.-M. Lin, D.-C. Wu, M.-H. Li and S.-P. Teng, Environ. Sci. Technol., 2010, 44, 5142–5247 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra19362e |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mMt = 0.78 mmol g−1 (1CEC), 1.56 mmol g−1 (2CEC), 2.34 mmol g−1 (3CEC), 3.12 mmol g−1 (4CEC). The obtained materials were accordingly defined as APTES1.0CEC–Mt, APTES2.0CEC–Mt, APTES3.0CEC–Mt, and APTES4.0CEC–Mt. Finally, the prepared products were dried in an oven, pulverized to pass through a 200 μm mesh sieve, and sealed for the next step.
mMt = 0.78 mmol g−1 (1CEC), 1.56 mmol g−1 (2CEC), 2.34 mmol g−1 (3CEC), 3.12 mmol g−1 (4CEC). The obtained materials were accordingly defined as APTES1.0CEC–Mt, APTES2.0CEC–Mt, APTES3.0CEC–Mt, and APTES4.0CEC–Mt. Finally, the prepared products were dried in an oven, pulverized to pass through a 200 μm mesh sieve, and sealed for the next step.
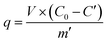




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kd
kd

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kd versus 1/T; R (8.314 J mol−1 K−1) is the gas law constant and T (K) is the absolute temperature. The thermodynamic parameters calculated are listed in Table 2. The positive values of ΔG for Mt, APTES1.0CEC–Mt and APTES2.0CEC–Mt indicate the non-spontaneous adsorption process, whereas the negative values for APTES3.0CEC–Mt and APTES4.0CEC–Mt indicate the spontaneous process. ΔH was positive for all materials, which means the adsorption processes are endothermic. Furthermore, the positive values of ΔS describe the increasing randomness in the solid/solution interface during the adsorption process.32
kd versus 1/T; R (8.314 J mol−1 K−1) is the gas law constant and T (K) is the absolute temperature. The thermodynamic parameters calculated are listed in Table 2. The positive values of ΔG for Mt, APTES1.0CEC–Mt and APTES2.0CEC–Mt indicate the non-spontaneous adsorption process, whereas the negative values for APTES3.0CEC–Mt and APTES4.0CEC–Mt indicate the spontaneous process. ΔH was positive for all materials, which means the adsorption processes are endothermic. Furthermore, the positive values of ΔS describe the increasing randomness in the solid/solution interface during the adsorption process.32

![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) XCa + Sr2+ ↔
XCa + Sr2+ ↔ ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) XSr + Ca2+
XSr + Ca2+
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SOH + Sr2+ ↔
SOH + Sr2+ ↔ ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SOSr + H+
SOSr + H+
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) R–NH2 + Sr2+ ↔
R–NH2 + Sr2+ ↔ ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) R–NH2Sr2+
R–NH2Sr2+






