Polyethyleneimine-stabilized hydroxyapatite nanoparticles modified with hyaluronic acid for targeted drug delivery†
Lijun Kong‡
a,
Zezhong Mu‡b,
Yuan Yua,
Lixia Zhanga and
Jinxia Hu*a
aDepartment of Biochemistry and Molecular Biology, Binzhou Medical University, Yantai, Shandong 264003, P. R. China. E-mail: hjxhope@163.com; Tel: +86-0535-6913241
bCheeloo Health Science Center, Shandong University, Jinan, Shandong 250000, P. R. China
First published on 14th October 2016
Abstract
Targeted delivery of therapeutic drugs into cancer cells is a facile method to improve therapeutic efficacy. Focused on this point, a functionalized porous hydroxyapatite (HAp) nanoparticle-based nanocarrier consisting of polyethyleneimine (PEI) coating and outer hyaluronic acid (HA) modification (HAp–PEI–HA) has been developed. In this nanocarrier, PEI was coated onto the HAp to stabilize the nanoparticles, and HA acted as an efficient targeting ligand to selectively bind the CD44 receptors which are overexpressed on the surface of some cancer cells. Various techniques demonstrated the successful preparation of the HAp–PEI–HA nanoparticles. The resulting HAp–PEI–HA nanoparticles showed better dispersibility and stability in aqueous solution compared to bare HAp. In addition, the DOX released from DOX@HAp–PEI–HA exhibited a pH-responsive release profile. The targeting property of HAp–PEI–HA was investigated on A549 cells with high CD44 receptor expression and U87 cells with low CD44 receptor expression. The result showed enhanced cellular uptake of HAp–PEI–HA nanoparticles in A549 cells through CD44 receptor mediated cellular endocytosis. Furthermore, the effective targeted delivery of DOX by HAp–PEI–HA in A549 cells led to enhanced therapeutic efficacy. Thus, the designed HAp–PEI–HA nanocarrier showed promising potential for targeted drug delivery in cancer therapy.
1. Introduction
Even though many newly emerged therapeutic approaches have been extensively explored, chemotherapy has still been considered as the dominant method to combat cancer. As a commonly used chemotherapeutic drug, doxorubicin (DOX) has always involved a large dosage and repeated administration due to its low tumor permeability and limited therapeutic index.1 To relieve the severe side effects of DOX and improve its therapeutic efficacy, various types of nanocarrier, including liposomes,2 nanogels,3 graphene oxide4 and mesoporous silica nanoparticles,5 have been widely explored for drug delivery systems of DOX, which could effectively protect DOX from binding with normal cells and accumulate in tumor sites due to the enhanced permeability and retention (EPR) effect.6 Thus, it is desirable to fabricate a versatile and biocompatible nanocarrier for safe and effective drug delivery.Hydroxyapatite (HAp) nanoparticles, a hydroxylated calcium phosphate-based material, have long been known as a preferable nanocarrier for its outstanding biocompatibility, non-immunogenicity and good drug-loading capacity.7 Moreover, the biodegradability of HAp also eliminates its safety issue and marks its superiority to other inorganic materials as an intracellular drug vehicle.8 For instance, Wang et al. used a hydrothermal method to synthesize porous hydroxyapatite with Pluronic F127 and CTAB as co-templates.9 They found that the obtained HAp could effectively load and release the hydrophobic drug carvedilol. Similarly, porous carbonated hydroxyapatite was also prepared by Ning et al. using a hydrothermal method without any template molecules for a drug delivery system.10 However, the morphology of the above HAp was non-uniform, which was likely due to the uncontrollable process in the reaction kettle. Alternatively, by precisely controlling the pH, precipitation temperature and molar ratio of precursor ions, porous HAp nanoparticles could be obtained through the coprecipitation method.11 But the bare HAp nanoparticles are always unstable in the physiological environment, which makes the surface coating of HAp very important.12 Therefore, it is meaningful to fabricate uniform porous HAp nanoparticles with a suitable surface coating shell for efficient intracellular drug delivery.
Branched polyethyleneimine (PEI), a low-cost commercially available polymer, has been frequently applied as a stabilizer to improve the physiological stability of various inorganic nanoparticles, such as gold nanoparticles,13 silver nanoparticles14 and Fe3O4 nanoparticles.15 Benefiting from its high density of amine groups and branched internal structure, PEI could render those nanoparticles excellent colloidal stability and dispersibility. However, the modification of PEI on HAp nanoparticles has rarely been reported to the best of our knowledge. Moreover, the severe toxicity and nonspecific cell uptake of PEI-coated nanoparticles still need to be carefully addressed. Shi et al. reported that the surface modification of branched PEI could effectively enhance its biocompatibility.16 Even though functionalized PEI with neutral and negative charges were nontoxic at concentrations up to 200 μg mL−1, the phagocytosis ratio of those PEI derivative coated nanoparticles may concurrently decline. Thus, a suitable ligand molecule needs to be employed to further modify PEI to reduce the cytotoxicity as well as enhance the cellular uptake of the nanoparticles. Hyaluronic acid (HA), composed of N-acetylglucosamine and a D-glucuronic acid disaccharide unit, has been regarded as a non-toxic and biodegradable natural acidic polysaccharide.17 One of the advantages of HA-coated nanoparticles is that the HA shell could commendably protect nanoparticles from the adhesion of plasma proteins, thus endowing the nanoparticles with good stability and circulation time. Besides, HA could significantly enhance the cancer cell uptake of nanoparticles due to its high affinity with the CD44 receptor, which is commonly overexpressed on several cancer cell surfaces.18 More interestingly, it has already been reported that CD44 receptors are highly expressed in the majority of non-small cell lung cancers (NSCLC) and it is a known biomarker for NSCLC tumors.19 Therefore, the further modification of HA on PEI-coated nanoparticles is probably beneficial for efficient nanocarrier-based drug delivery.
Hence, we hypothesized that HA conjugated PEI-stabilized HAp nanoparticles could effectively deliver DOX into A549 lung cancer cells for enhanced therapeutic efficacy. The uniform rod-like HAp nanoparticles were first prepared and then stabilized by PEI to form a stable colloidal solution. Lastly, the targeting ligand HA was attached onto the PEI-stabilized HAp nanoparticles (HAp–PEI–HA) and the model drug DOX was loaded into the HAp–PEI–HA (DOX@HAp–PEI–HA). The physiochemical properties and stability of the nanoparticles were successively characterized. The drug loading and releasing behavior of HAp–PEI–HA was also investigated. The cytotoxicity of the hybrid nanoparticles was evaluated and a hemolysis assay was conducted to assess the hemocompatibility. Furthermore, the targeting ability of HAp–PEI–HA towards A549 cells was also demonstrated. The cellular uptake behavior and therapeutic efficacy of DOX@HAp–PEI–HA were also investigated.
2. Experimental section
2.1. Materials
Calcium nitrate (Ca(NO3)2·4H2O), ammonium phosphate ((NH4)2HPO4) and ammonia solution (NH3·H2O) were purchased from Aladdin Chemistry, Co., Ltd. (Shanghai, China). 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl), N-hydroxysuccinimide (NHS) and polyethyleneimine (PEI, Mw = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were supplied by Sigma-Aldrich Trading Co., Ltd. (Shanghai, China). Hyaluronic acid (HA, Mw = 31
000) were supplied by Sigma-Aldrich Trading Co., Ltd. (Shanghai, China). Hyaluronic acid (HA, Mw = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) was provided by Zhenjiang Dong Yuan Biotechnology Corporation (Zhenjiang, China). Dulbecco’s modified Eagle’s medium, fetal bovine serum (FBS), penicillin, streptomycin and phosphate buffer saline (PBS) were obtained from Gibco (Grand Island, USA). Cell Counting Kit-8 (CCK-8) was purchased from Beyotime Institute of Biotechnology (Shanghai, China). 4′,6-Diamidino-2-phenylindole (DAPI) and fluorescein isothiocyanate (FITC) were received from Yeasen Biotech Co., Ltd. (Shanghai, China). The water used in all experiments was purified by a Milli-Q water purification system (Millipore, Bedford, MA) with a resistivity of 18.2 MΩ cm. All other chemicals were of analytical grade from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
200) was provided by Zhenjiang Dong Yuan Biotechnology Corporation (Zhenjiang, China). Dulbecco’s modified Eagle’s medium, fetal bovine serum (FBS), penicillin, streptomycin and phosphate buffer saline (PBS) were obtained from Gibco (Grand Island, USA). Cell Counting Kit-8 (CCK-8) was purchased from Beyotime Institute of Biotechnology (Shanghai, China). 4′,6-Diamidino-2-phenylindole (DAPI) and fluorescein isothiocyanate (FITC) were received from Yeasen Biotech Co., Ltd. (Shanghai, China). The water used in all experiments was purified by a Milli-Q water purification system (Millipore, Bedford, MA) with a resistivity of 18.2 MΩ cm. All other chemicals were of analytical grade from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Fabrication of HAp nanoparticles
Porous HAp nanoparticles were prepared using a modified chemical coprecipitation method according to the previous report.20 Briefly, an ethanol solution of Ca(NO3)2 (12.5 mmol, 50 mL) was prepared and continuously stirred in a conical flask. Subsequently, ammonia solution was added slowly into the solution and the pH was adjusted to 10–11. Afterwards, the aqueous solution of (NH4)2HPO4 (12.5 mmol, 30 mL) was then added dropwise to the alkalized Ca(NO3)2 solution with vigorous stirring. The resulting suspension was stirred accompanied with sustained ammonia solution addition (20 mL h−1) at 80 °C for 6 h. After cooling down at room temperature, the original precipitate was rinsed using deionized water mixed with ethanol (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and ultrapure water three times, respectively. The porous HAp was obtained by centrifugation (8000 rpm) and freeze dried for 24 h.
1) and ultrapure water three times, respectively. The porous HAp was obtained by centrifugation (8000 rpm) and freeze dried for 24 h.
2.3. Fabrication of HAp–PEI and HAp–PEI–HA nanoparticles
The fabrication of HAp–PEI followed the previous description.21 200 mg of HAp was ultrasonically dispersed in 20 mL of ultrapure water. PEI (0.1 g) dissolved in 1 mL of water was then added into the above aqueous solution of HAp (20 mL). Subsequently, the mixture was sonicated for 30 min, and then stirred at room temperature for 24 h. After purification by 3 cycles of centrifugation (8000 rpm, 10 min)/redispersion in water, the HAp–PEI was obtained for further experiments.The HAp–PEI–HA was synthesized from the HAp–PEI. The HA was chemically conjugated to HAp–PEI after being activated by EDC and NHS. At first, 22 mg of EDC, 18 mg of NHS and 0.1 g of HA were dissolved in 10 mL of DMSO. Subsequently, 0.2 g of HAp–PEI dispersed in 10 mL of DMSO was slowly added. After stirring for 24 h, the reaction was terminated by the addition of 20 mL of purified water. The product was collected and purified by centrifugation. Ultimately, the obtained HAp–PEI–HA was suspended in ultrapure water for further use.
2.4. Characterization
The morphology of the as-prepared nanoparticles was examined using transmission electron microscopy (TEM, JEOL 2010F, Japan) with an operating voltage of 200 kV. All TEM samples were prepared by dropping the particle suspension onto a carbon-coated copper grid and air drying before taking measurements. The chemical structure of the composite was confirmed by Fourier transform infrared (FTIR) spectra using a Nicolet Nexus 670 FTIR spectrophotometer (Thermo Nicolet Corporation, USA) with a 500–4500 cm−1 infrared range. Phase analysis was conducted via X-ray diffraction (XRD) with a D/max 2550 PC X-ray diffractometer (Rigaku Corp., Japan). To evaluate the surface-modified degree of HAp, thermal gravimetric analysis (TGA) was performed using a TG 209F1 (NETZSCH Instruments Co., Ltd., Germany) thermal gravimetric analyzer at a heating rate of 10 °C min−1 under N2 atmosphere. The zeta-potentials were determined using Zetasizer Nano ZS apparatus (Malvern Instruments, UK). UV-vis spectroscopy was carried out using a Lambda 25 UV-vis spectrophotometer (PerkinElmer, Boston, MA) and the samples were dispersed in water before measurements. The specific surface area and pore size distribution were measured using a Micromeritics surface area and porosity analyzer with a degas temperature of 105 °C and an outgas time of 12 h.2.5. DOX loading and in vitro release
To load DOX into the as-prepared nanoparticles, 20 mg of HAp–PEI–HA was mixed with 2 mL of DOX solution (10 mg mL−1). The resulting suspension was stirred at room temperature for 24 h. Afterwards, the nanoparticles were collected through centrifugation at 8000 rpm and washed repeatedly with deionized water to harvest the DOX-loaded HAp–PEI–HA.For in vitro release of DOX from HAp–PEI–HA, the DOX@HAp–PEI–HA was respectively dissolved in 2 mL of pH 7.4 and 5.0 buffer solutions and transferred into dialysis bags (cut-off molecular weight = 3500). Then the dialysis bags were immersed in 9 mL of the corresponding buffer solutions with gentle shaking at 37 °C. At designed time intervals, 2 mL of solution was withdrawn from the suspension, after which the same volume of fresh buffer solution was added into the system. The released DOX in the supernatant was then determined using an ultraviolet-visible (UV-vis) spectrophotometer at a fixed wavelength of 480 nm.
2.6. Cell culture
A549 cells (human alveolar basal epithelial cells) and U87 cells (human glioblastoma cell line) bought from American Type Culture Collection (ATCC, Rockville, MD) were selected as test cells. The cells were grown using DMEM supplemented with 10% fetal bovine serum, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin at 37 °C in a humidified incubator with 5% CO2.2.7. Cell viability assay
Cell viability was determined using the Cell Counting Kit-8 assay following the supplier’s instruction. During the experiment, A549 cells were firstly seeded in 96-well plates with a density of 5 × 103 cells per well in growth medium and cultured for 24 h prior to the exposure to the test materials. Subsequently, A549 cells were cultured in the growth medium containing different concentrations (12.5, 25, 50, 100 and 200 μg mL−1) of HAp and HAp–PEI–HA nanoparticles, respectively, for 24 h. Meanwhile, the wells only containing cell medium were used as controls. At the end of the treatment, the medium was removed and the cells were washed with PBS three times. Then the serum-free medium was added and 10 μL of CCK-8 solution was added to each well and the plates were incubated for another 1 h at standard culture conditions. The absorbance of each well was measured through single wavelength spectrophotometry at 450 nm with a microplate reader. The experimental results were averaged by three measurements. The relative cell viability could be quantitatively calculated using the equation as follows.2.8. Hemolysis assay
A hemolysis assay of the nanoparticles was performed according to protocols described in the literature.22 Briefly, fresh blood was withdrawn from a rabbit’s auricular vein with heparin as an anticoagulant. The blood was then centrifuged at 2000 rpm for 5 min, and diluted 10-fold with normal saline to obtain rabbit red blood cells (RBCs). Subsequently, the diluted RBC suspension was further mixed with various substances. 0.1 mL of diluted RBC suspension mixed with 0.9 mL of deionized water was used as a positive control, whereas it blended with 0.9 mL of PBS was set as a negative control. The same suspension was also added into 0.9 mL of PBS containing HAp or HAp–PEI–HA at different concentrations (12.5, 25, 50, 100, 200, 400 and 800 μg mL−1). These mixtures were gently shaken at room temperature for 3 h. Afterward, all samples were centrifuged at 5000 rpm for 10 min. The absorbance of the supernatants (hemoglobin) was determined using a UV-vis spectrophotometer at 541 nm. The hemolysis percentage was calculated according to the previous report.212.9. Cellular uptake of HAp–PEI–HA and DOX@HAp–PEI–HA
To evaluate the targeting effect of HAp–PEI–HA, the HAp–PEI–HA was labeled with FITC according to the previous report23 and the A549 and U87 cells were employed as model cells. Briefly, the A549 or U87 cells were seeded onto slides in 6-well culture plates with a density of 105 cells per well. After being cultured overnight at 37 °C under 5% CO2, the cells were washed with PBS three times and then co-cultured with FITC-labeled HAp–PEI–HA with a concentration of 50 μg mL−1 for 4 h. To further demonstrate the targeted uptake of HAp–PEI–HA nanoparticles, a competitive inhibition assay was conducted by pre-treatment of A549 cells with free HA molecules for 3 h to block the receptor. Subsequently, the cells were rinsed using PBS and fixed with 1.5 mL of 4% paraformaldehyde at 4 °C overnight. DAPI was then added into the fixed cells to stain the nuclei. Finally, samples were observed using confocal microscopy. The cell uptake of DOX@HAp–PEI–HA in A549 against free DOX was performed by a similar protocol as for HAp–PEI–HA.2.10. Antitumor activity evaluation
The in vitro antitumor activity of DOX@HAp–PEI–HA was assessed against A549 cells. The experimental process is similar to the cell viability assay. About 8000 A549 cells were cultured in 100 μL of medium per well in a 96-well plate for 24 h to allow the cells’ attachment on the plate. The seeded cells were then treated with free DOX and DOX@HAp–PEI–HA at different DOX concentrations (0.05, 0.1, 0.2, 0.5, 1, and 2 μg mL−1). After incubation for 24 h, the medium was removed and replaced by the CCK-8 agent for another 1 h incubation. At the end of the incubation, the absorbance of the solution was recorded at 450 nm using a microplate reader and the cell viability was calculated according to the above mentioned equation.Also, the inhibition experiment was performed to clarify the targeted therapeutic efficacy of DOX@HAp–PEI–HA using a similar protocol as previous reports.24,25 In brief, the A549 cells were incubated with free DOX and DOX@HAp–PEI–HA at various DOX concentrations for 4 h. After that, the medium was replaced with fresh complete medium in the absence of DOX. The cells were cultured at 37 °C for another 44 h. Lastly, the cell viability was tested by CCK-8 assay.
2.11. Live subject statement
In this work, human subjects were not involved for any experimentation. All animal studies were performed in compliance with the guidelines of the US National Institutes of Health and approved by the Experimental Animal Center of Binzhou Medical University.2.12. Statistical analysis
All values were presented as mean ± standard deviation. Statistical analysis was carried out by the one-way analysis of variance (one-way ANOVA) and Scheffe’s post hoc test. *P < 0.05 and **P < 0.01 were considered statistically significant.3. Results and discussion
3.1. Characterization of nanoparticles
The synthetic route for the functionalized HAp is shown in Fig. 1. The porous HAp nanoparticles were first fabricated via a co-precipitation method.26 PEI molecules were then physically adsorbed onto the surface of HAp to improve their stability and used as a linker for further modification. Subsequently, hyaluronic acid was grafted onto HAp–PEI by the reaction between PEI and HA through NHS/EDC chemistry. The HA modification could enable the obtained HAp–PEI–HA to target CD44-overexpressing A549 cells. Furthermore, by loading the anticancer drug, the drug-loaded HAp–PEI–HA was employed to effectively deliver the anticancer drug into CD44 positive A549 cells for tumor therapy.The morphology of the fabricated nanoparticles was characterized using TEM. As shown in Fig. 2A, the HAp nanoparticles showed typical rod-like morphology with a measured particle size of about 150 nm. In addition, numerous pores on the surface of the HAp can also be observed, which is in accordance with the result in the referenced literature.26 After functionalization with PEI and HA, there was no obvious change in the morphology and structure (Fig. 2B). However, it was clearly seen that the resulting HAp–PEI–HA nanoparticles were relatively indistinct, indicating the successful functionalization of PEI–HA. We also can observe the polymeric shell on the surface of HAp–PEI–HA, with a thickness of around 3.5 nm. Fig. 2C shows the XRD pattern of HAp. The marked XRD peaks of the HAp sample were found to be consistent with the standard hydroxyapatite phase (JCPDS card no. 09-432), confirming the successful fabrication of pure HAp. The N2 adsorption–desorption isotherms of HAp and HAp–PEI–HA are presented in Fig. 2D. Both HAp and HAp–PEI–HA samples give classical type-IV isotherms, indicating the mesoporous character of the nanoparticles. Furthermore, the hysteresis loops of type H1 appeared as almost vertical and nearly parallel at the relative pressure (P/P0) of 0.9–1.0, revealing relatively uniform cylindrical like pores in the HAp nanorods.27 This result is similar to previous TEM observations.
 | ||
| Fig. 2 TEM images of (A) HAp and (B) HAp–PEI–HA. (C) XRD pattern of HAp. (D) N2 adsorption–desorption isotherm of HAp and HAp–PEI–HA. | ||
Next, we characterized the successful preparation of HAp–PEI–HA nanoparticles using various techniques, including TGA, FTIR spectra and zeta potential measurement. From the results of TGA in Fig. 3A, when the temperature reached 900 °C, the residual amounts were found to be 96.07 wt%, 90.34 wt% and 79.38 wt% for the HAp, HAp–PEI and HAp–PEI–HA samples, respectively. Thus, the amount of PEI on the HAp–PEI–HA sample can be calculated to be about 5.73 wt%, and HA about 10.96 wt%. This result suggested that HA was successfully grafted onto HAp by the assistance of PEI. Fig. 3B shows the FTIR spectra of HAp, HAp–PEI and HAp–PEI–HA samples. Compared with bare HAp, the characteristic absorption peaks at 2957 and 2844 cm−1 assigned to C–H stretching vibrations appeared in the HAp–PEI sample.28 In addition, new peaks at 1557 and 1468 cm−1 attributed to N–H bending vibration also appeared, which indicated the successful coating of PEI.29 Furthermore, the successful grafting of HA onto HAp–PEI was confirmed by the appearance of absorption peaks at 1645 cm−1 in the sample of HAp–PEI–HA, which could be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in the amide groups.30 The zeta potential data are displayed in Fig. 3C. The zeta potential of bare HAp nanoparticles showed a relatively low absolute value (3.4 mV), whereas they became more negatively charged at pH 9.0 (Fig. S1†), which was beneficial for the absorption of PEI taking account of the alkalinity of the PEI aqueous solution. It had been reported that HAp was more negative with the increased pH value,31 thus we deduced that the PEI would be readily attached onto the HAp surface to form stable hybrid nanoparticles through the electrostatic interaction. We can observe that the value of zeta potential in HAp–PEI was increased to 32.7 mV after PEI coating due to the presence of the high density of amine groups. After conjugation of HA, the zeta potential of HAp–PEI–HA decreased to −31.1 mV, which indicated the existence of large numbers of carboxyl groups. Therefore, the changed surface charge further confirmed the successful preparation of HAp–PEI–HA nanoparticles. According to the above results, we concluded that HAp–PEI–HA had been successfully fabricated.
O stretching vibration in the amide groups.30 The zeta potential data are displayed in Fig. 3C. The zeta potential of bare HAp nanoparticles showed a relatively low absolute value (3.4 mV), whereas they became more negatively charged at pH 9.0 (Fig. S1†), which was beneficial for the absorption of PEI taking account of the alkalinity of the PEI aqueous solution. It had been reported that HAp was more negative with the increased pH value,31 thus we deduced that the PEI would be readily attached onto the HAp surface to form stable hybrid nanoparticles through the electrostatic interaction. We can observe that the value of zeta potential in HAp–PEI was increased to 32.7 mV after PEI coating due to the presence of the high density of amine groups. After conjugation of HA, the zeta potential of HAp–PEI–HA decreased to −31.1 mV, which indicated the existence of large numbers of carboxyl groups. Therefore, the changed surface charge further confirmed the successful preparation of HAp–PEI–HA nanoparticles. According to the above results, we concluded that HAp–PEI–HA had been successfully fabricated.
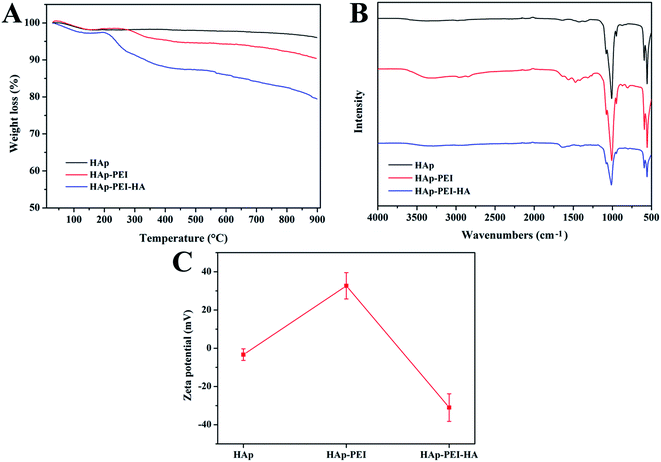 | ||
| Fig. 3 (A) TGA curves, (B) FTIR spectra and (C) zeta potential of HAp, HAp–PEI and HAp–PEI–HA in deionized water at room temperature. | ||
The stability of nanoparticles is a critical factor to affect their practical application in biological systems. As shown in Fig. 4A, the hydrodynamic size of HAp, HAp–PEI and HAp–PEI–HA samples measured using DLS were 1275.3, 246.17 and 270.5 nm, respectively. There was no obvious change in particle size for HAp–PEI and HAp–PEI–HA samples. But HAp exhibited a much larger size than both HAp–PEI and HAp–PEI–HA. This result indicated that HAp–PEI and HAp–PEI–HA possessed better dispersibility in water than HAp due to the existence of hydrophilic polymers. To further confirm the stability of functionalized HAp, all the samples were suspended in aqueous solution and then photos were taken after storage for different amounts of time (Fig. 4B). It can be clearly seen that bare HAp nanoparticles showed obvious sedimentation at the bottom of the tube in a short amount of time (10 min). Notably, HAp–PEI and HAp–PEI–HA were still unexpectedly stable when suspended for 120 min, suggesting the outstanding colloidal stability of HAp–PEI–HA. These results convinced us that the PEI molecule could sufficiently stabilize the HAp nanoparticles and the modification of HA did not affect the stability of the hybrid nanoparticles, which is likely due to the relatively high value of zeta potential for HAp–PEI and HAp–PEI–HA.
 | ||
| Fig. 4 (A) Size distributions and (B) stability in water for different times of (a–c) HAp, HAp–PEI and HAp–PEI–HA. | ||
3.2. In vitro DOX loading and release
The anticancer drug DOX was used as the model drug to be loaded into the resulting HAp–PEI–HA nanoparticles. The loading efficiency was calculated to be 3.9%. After DOX loading, the DOX@HAp–PEI–HA suspension solution was reddish and showed the characteristic absorption band at 480 nm of DOX (Fig. 5A). In addition, the hydrodynamic size of DOX@HAp–PEI–HA determined by DLS was 275.8 nm (Fig. S2†). After centrifugation, no absorption band appeared in the supernatant, which indicated the stable encapsulation of DOX in HAp–PEI–HA nanoparticles. | ||
| Fig. 5 (A) UV-vis spectra of HAp–PEI–HA nanoparticles suspended in water before/after centrifugation. (B) The cumulative release profiles of DOX from DOX@HAp–PEI–HA under different conditions. | ||
To detect the in vitro DOX release behavior, DOX@HAp–PEI–HA nanoparticles were subjected to buffer solutions of different pH values at 37 °C. As clearly shown in Fig. 5B, the cumulative DOX release percentage from DOX@HAp–PEI–HA at pH 7.4 was only 23% within 48 h, while more than 88% DOX release was observed at pH 5.0. Thus, DOX released from DOX@HAp–PEI–HA was pH-dependent. Under acidic conditions, the amino of DOX was protonated, weakening the adsorption between HAp nanoparticles and DOX.32 Meanwhile, PEI was swelled in the acidic environment due to the protonation of amine groups.33 Therefore, these reasons strongly contributed to facilitate the diffusion of DOX out of the DOX@HAp–PEI–HA in acidic conditions. As a result, this pH-responsive drug release property of DOX@HAp–PEI–HA nanoparticles makes them promising for cancer treatment, since tumor tissue and tumor cells are mildly acidic.34
3.3. Biocompatibility evaluation
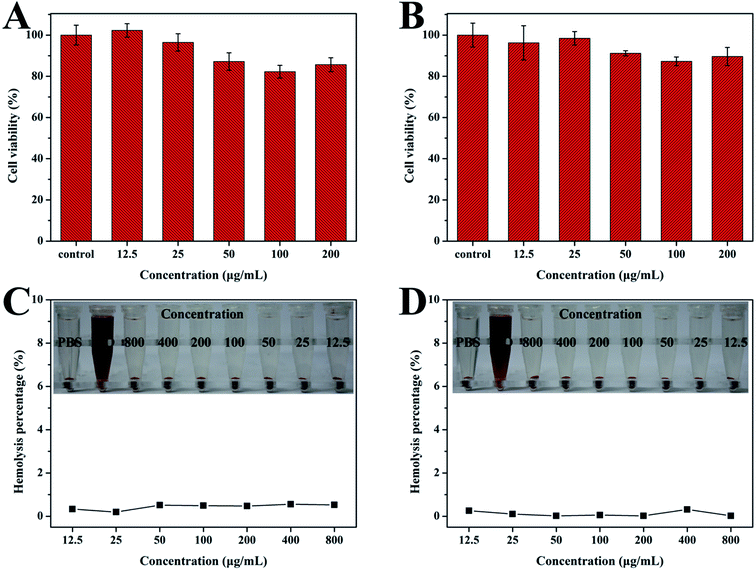
Before evaluating the anticancer efficacy of DOX@HAp–PEI–HA, we investigated the in vitro cytotoxicity of HAp–PEI–HA via the CCK-8 assay. It was observed that the cytotoxicity of both bare HAp and HAp–PEI–HA nanoparticles were dose-dependent (Fig. 6A and B). When the particle concentration was 200 μg mL−1, the bare HAp showed reduced cell viability, with cell viability of 85.6% (Fig. 6A). In contrast, HAp–PEI–HA exhibited relatively higher cell viability (89.7%) compared to the HAp sample at the same concentration (Fig. 6B). To further study the compatibility of the as-prepared nanoparticles, the hemolysis assay was performed in vitro. As depicted in Fig. 6C and D, there was no visible hemoglobin release and detected hemolytic activity for HAp and HAp–PEI–HA samples even at the tested particle concentration of 800 μg mL−1. These findings indicated that the HAp–PEI–HA nanoparticles had good biocompatibility.3.4. Cellular uptake and drug delivery
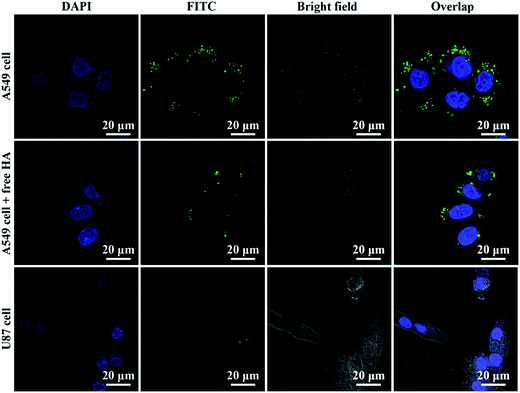
Targeted delivery of therapeutic drugs into cancer cells is an important aspect to improve therapeutic efficacy. It was reported that A549 is a human alveolar basal epithelial cell line with high expression of CD44, while U87 is a human glioblastoma cell line with low expression of CD44.19,35 Thus, we investigated the targeting ability of the HAp–PEI–HA nanocarrier by incubation with the two kinds of cell lines. The internalized nanoparticles with green fluorescence (FITC labeled HAp–PEI–HA) in cells could be clearly seen from the CLSM images, as shown in Fig. 7. For A549 cells, it was distinctly observed that many green fluorescence dots appeared within the cells and were distributed around the nucleus. In comparison, much fewer green fluorescence dots can be seen for the negative U87 cells. Moreover, the competitive inhibition assay was conducted to demonstrate the specific binding ability of HAp–PEI–HA with positive A549 cells. It turned out that the presence of free HA could apparently subdue the cellular uptake of HAp–PEI–HA nanoparticles, as evidenced by the attenuated green fluorescence in the inhibited group. Those results suggested that HAp–PEI–HA nanoparticles with HA modification had the ability to target CD44-overexpressed cancer cells, and the specific affinity between HA and the CD44 receptor can facilitate intracellular uptake of HAp–PEI–HA. Subsequently, the DOX delivery by HAp–PEI–HA was investigated by CLSM observation in A549 cells. As displayed in Fig. 8, the red fluorescence indicated the DOX. After 4 h incubation, the red fluorescence was overlapped with the blue fluorescence, indicating that the free DOX rapidly diffused into the cell nucleus. For the DOX@HAp–PEI–HA group, it was clearly seen that a significant amount of DOX@HAp–PEI–HA nanoparticles with DOX fluorescence resided in the A549 cells and were mainly distributed in the cytoplasm. The CLSM observation demonstrated the efficient drug delivery by HAp–PEI–HA nanoparticles due to the efficient targeting effect of HA on the HAp–PEI–HA. | ||
| Fig. 8 CLSM images of A549 cells after 4 h incubation with free DOX and DOX@HAp–PEI–HA nanoparticles. | ||
3.5. Antitumor activity assay
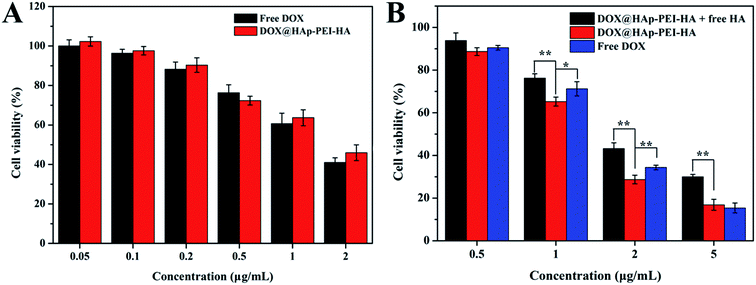
To evaluate the antitumor activity of the DOX-loaded nanocarrier, the in vitro cytotoxicity against A549 cells was studied using the CCK-8 assay. The cell viabilities of A549 cells after treatment with free DOX and DOX@HAp–PEI–HA nanoparticles at different DOX concentrations (0.05, 0.1, 0.2, 0.5, 1 and 2 μg mL−1) for 24 h are presented in Fig. 9. The result showed a dose-dependent increase in cytotoxicity for both free DOX and DOX@HAp–PEI–HA groups. For instance, the cell viability of A549 cells treated with DOX@HAp–PEI–HA was 102.3%, 97.6%, 90.3%, 72.3%, 63.8% and 46.0% at the tested DOX concentrations, respectively. Under the same DOX concentration, there was no significant difference in the cytotoxic effect between free DOX and DOX@HAp–PEI–HA. At the DOX concentration of 2 μg mL−1, the cell viability was reduced to 42.0% and 46.0% for free DOX and DOX@HAp–PEI–HA, respectively. Although the loaded DOX was not completely released from DOX@HAp–PEI–HA in 24 h, it still caused a considerable level of cytotoxicity compared with free DOX. On the other hand, as previous reports suggested that short time incubation with drug-loaded nanocarriers was more favorable to clarify the targeted anticancer therapeutic efficacy of the nanocarrier,24,25 A549 cells were incubated with free DOX and DOX@HAp–PEI–HA for 4 h, followed by removal of the unbounded nanocarrier. Meanwhile, the inhibition assay was also conducted by pre-incubation with free HA. Expectedly, the DOX@HAp–PEI–HA showed obviously enhanced cytotoxicity compared to the inhibition group and free DOX group at the DOX concentrations of 1 and 2 μg mL−1, indicating that the targeting ability of HA indeed improved the therapeutic efficacy of DOX@HAp–PEI–HA. This result was probably because targeted delivery of DOX in A549 cells by HAp–PEI–HA was more effective at entering the cells than the inhibition group and free DOX group, resulting in more DOX accumulating in the cells and eventually causing obvious cytotoxicity. Consequently, the results demonstrated that HAp–PEI–HA nanoparticles are promising for application as a drug carrier in cancer treatment.4. Conclusions
In summary, a functionalized HAp–PEI–HA nanocarrier with specific targeting ability was fabricated. The resulting HAp–PEI–HA nanoparticles were stable and dispersed in aqueous solution. The anticancer drug DOX released from DOX-loaded HAp–PEI–HA in a pH-responsive release manner. As evidenced by the cell viability assay and hemolysis assay, the functionalized nanocarrier showed good biocompatibility. The targeting effect of HAp–PEI–HA nanoparticles through CD44 receptor mediated endocytosis was demonstrated by incubation with CD44 receptor high-expressing A549 cells and CD44 receptor low-expressing U87 cells. As a result, much more cellular uptake of HAp–PEI–HA nanoparticles in A549 cells was observed using confocal microscopy. Moreover, the efficient intracellular delivery of DOX by HAp–PEI–HA resulted in high therapeutic effect. Our results indicated that the constructed targeting delivery system holds the potential for cancer treatment.Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (No. 81302017) and Shandong Provincial Natural Science Foundation, China (No. ZR2013HL004).Notes and references
- A. I. Minchinton and I. F. Tannock, Nat. Rev. Cancer, 2006, 6, 583–592 CrossRef CAS PubMed.
- Q. Chen, H. W. Ding, J. X. Zhou, X. F. Zhao, J. L. Zhang, C. R. Yang, K. X. Li, M. X. Qiao, H. Y. Hu, P. T. Ding and X. L. Zhao, RSC Adv., 2016, 6, 17782–17791 RSC.
- D. Das, P. Patra, P. Ghosh, A. P. Rameshbabu, S. Dhara and S. Pal, Polym. Chem., 2016, 7, 2965–2975 RSC.
- F. Nasrollahi, J. Varshosaz, A. A. Khodadadi, S. Lim and A. Jahanian-Najafabadi, ACS Appl. Mater. Interfaces, 2016, 8, 13282–13293 CAS.
- W. Feng, X. J. Zhou, C. L. He, K. X. Qiu, W. Nie, L. Chen, H. S. Wang, X. M. Mo and Y. Z. Zhang, J. Mater. Chem. B, 2013, 1, 5886–5898 RSC.
- S. G. Wang, Y. L. Wu, R. Guo, Y. P. Huang, S. H. Wen, M. W. Shen, J. H. Wang and X. Y. Shi, Langmuir, 2013, 29, 5030–5036 CrossRef CAS PubMed.
- B. Palazzo, M. Iafisco, M. Laforgia, N. Margiotta, G. Natile, C. L. Bianchi, D. Walsh, S. Mann and N. Roveri, Adv. Funct. Mater., 2007, 17, 2180–2188 CrossRef CAS.
- Y. H. Yang, C. H. Liu, Y. H. Liang, F. H. Lin and K. C. W. Wu, J. Mater. Chem. B, 2013, 1, 2447–2450 RSC.
- Q. F. Zhao, T. Y. Wang, J. Wang, L. Zheng, T. Y. Jiang, G. Cheng and S. L. Wang, Appl. Surf. Sci., 2011, 257, 10126–10133 CrossRef CAS.
- Y. P. Guo, L. H. Guo, Y. B. Yao, C. Q. Ning and Y. J. Guo, Chem. Commun., 2011, 47, 12215–12217 RSC.
- V. Uskokovic and D. P. Uskokovic, J. Biomed. Mater. Res., Part B, 2011, 96, 152–191 CrossRef PubMed.
- G. D. Venkatasubbu, S. Ramasamy, G. S. Avadhani, V. Ramakrishnan and J. Kumar, Powder Technol., 2013, 235, 437–442 CrossRef CAS.
- S. H. Wen, F. Y. Zheng, M. W. Shen and X. Y. Shi, Colloids Surf., A, 2013, 419, 80–86 CrossRef CAS.
- C. Aymonier, U. Schlotterbeck, L. Antonietti, P. Zacharias, R. Thomann, J. C. Tiller and S. Mecking, Chem. Commun., 2002, 3018–3019 RSC.
- H. D. Cai, X. An, J. Cui, J. C. Li, S. H. Wen, K. G. Li, M. W. Shen, L. F. Zheng, G. X. Zhang and X. Y. Shi, ACS Appl. Mater. Interfaces, 2013, 5, 1722–1731 CAS.
- S. H. Wen, F. Y. Zheng, M. W. Shen and X. Y. Shi, J. Appl. Polym. Sci., 2013, 128, 3807–3813 CrossRef CAS.
- T. Y. Jiang, Z. H. Zhang, Y. L. Zhang, H. X. Lv, J. P. Zhou, C. C. Li, L. L. Hou and Q. Zhang, Biomaterials, 2012, 33, 9246–9258 CrossRef CAS PubMed.
- V. M. Platt and F. C. Szoka, Mol. Pharmaceutics, 2008, 5, 474–486 CrossRef CAS PubMed.
- S. Ganesh, A. K. Iyer, D. V. Morrissey and M. M. Amiji, Biomaterials, 2013, 34, 3489–3502 CrossRef CAS PubMed.
- L. N. Gu, X. M. He and Z. Y. Wu, Mater. Chem. Phys., 2014, 148, 153–158 CrossRef CAS.
- J. C. Li, Y. Hu, J. Yang, P. Wei, W. J. Sun, M. W. Shen, G. X. Zhang and X. Y. Shi, Biomaterials, 2015, 38, 10–21 CrossRef CAS PubMed.
- Y. Hu, J. Yang, P. Wei, J. C. Li, L. Ding, G. X. Zhang, X. Y. Shi and M. W. Shen, J. Mater. Chem. B, 2015, 3, 9098–9108 RSC.
- J. C. Li, Y. He, W. J. Sun, Y. Luo, H. D. Cai, Y. Q. Pan, M. W. Shen, J. D. Xia and X. Y. Shi, Biomaterials, 2014, 35, 3666–3677 CrossRef CAS PubMed.
- F. Y. Zhou, B. Feng, H. J. Yu, D. G. Wang, T. T. Wang, J. P. Liu, Q. S. Meng, S. L. Wang, P. C. Zhang, Z. W. Zhang and Y. P. Li, Theranostics, 2016, 6, 679–687 CrossRef CAS PubMed.
- G. X. Chen, D. Li, J. C. Li, X. Y. Cao, J. H. Wang, X. Y. Shi and R. Guo, New J. Chem., 2015, 39, 2847–2855 RSC.
- E. Iyyappan, P. Wilson, K. Sheela and R. Ramya, Mater. Sci. Eng., C, 2016, 63, 554–562 CrossRef CAS PubMed.
- M. Thommes, Chem. Ing. Tech., 2010, 82, 1059–1073 CrossRef CAS.
- L. Yuan, Q. Q. Tang, D. Yang, J. Z. Zhang, F. Y. Zhang and J. H. Hu, J. Phys. Chem. C, 2011, 115, 9926–9932 CAS.
- X. Du, B. Y. Shi, J. Liang, J. X. Bi, S. Dai and S. Z. Qiao, Adv. Mater., 2013, 25, 5981–5985 CrossRef CAS PubMed.
- Z. W. Chen, Z. H. Li, Y. H. Lin, M. L. Yin, J. S. Ren and X. G. Qu, Chem.–Eur. J., 2013, 19, 1778–1783 CrossRef CAS PubMed.
- Y. Z. Zhao, M. Yang, H. B. Zhang, J. Zhu and K. C. Zhou, J. Cent. South Univ., 2016, 23, 1548–1555 CrossRef CAS.
- H. Xiong, S. Du, J. Ni, J. P. Zhou and J. Yao, Biomaterials, 2016, 94, 70–83 CrossRef CAS PubMed.
- X. Ma, Y. Zhao, K. W. Ng and Y. L. Zhao, Chem.–Eur. J., 2013, 19, 15593–15603 CrossRef CAS PubMed.
- H. Mok, O. Veiseh, C. Fang, F. M. Kievit, F. Y. Wang, J. O. Park and M. Q. Zhang, Mol. Pharmaceutics, 2010, 7, 1930–1939 CrossRef CAS PubMed.
- H. J. Cho, H. Y. Yoon, H. Koo, S. H. Ko, J. S. Shim, J. H. Lee, K. Kim, I. C. Kwon and D. D. Kim, Biomaterials, 2011, 32, 7181–7190 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra19351j |
| ‡ Lijun Kong and Zezhong Mu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |