Amorphous NiB/carbon nanohybrids: synthesis and catalytic enhancement induced by electron transfer†
Abstract
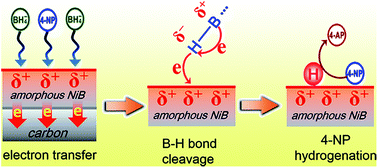
Catalytic conversion of hazardous 4-nitrophenol (4-NP) to 4-aminophenol over cost-effective catalysts is environmentally and commercially desired. Herein, we have synthesized amorphous NiB/C nanohybrids through a solution-phase reduction using pre-oxidized carbon nanoparticles as support. The amorphous catalyst shows promising activity towards the reduction of 4-NP with a high kn (3.640 s−1 mg−1) and low activation energy (35.4 kJ−1 mol−1). The catalytic activity highly depends on the carbon support and the crystalline degree of NiB alloy. An electron-transfer-induced catalytic mechanism is proposed to explain the catalytic performance of NiB/C. It is found that the electron transfer in metal/support interfaces makes NiB alloy electrophilic, favoring the adsorption of reactants, generation of active hydrogen and hydrogenation of 4-NP. The amorphous NiB/C catalyst can be recycled five times without obvious loss of activity. Our work demonstrates an example to explore the catalytic behavior of amorphous NiB/C catalyst, which may shed light on developing other advanced amorphous catalysts for 4-NP reduction.

 Please wait while we load your content...
Please wait while we load your content...