The critical zeta potential of polymer membranes: how electrolytes impact membrane fouling†
D. Breite,
M. Went,
A. Prager and
A. Schulze*
Leibniz Institute of Surface Modification, Permoserstraße 15, Leipzig, D-04318, Germany. E-mail: agnes.schulze@iom-leipzig.de; Tel: +49 341 235 2400
First published on 10th October 2016
Abstract
The zeta potential of membrane surfaces and the resulting electrostatic interactions are determining factors of membrane fouling. This study investigates the influence of environmental parameters like pH value, salt concentration, or ion valence on the zeta potential of polymer membranes and the resulting fouling. To control electrostatic forces charged polystyrene beads were used as fouling reagents. Also, polyethersulfone and polyvinylidene fluoride membranes were modified to possess an either positive or negative surface charge. Afterwards, suspensions of beads were filtered through the membranes in different electrolytic environments. Fouling occurred when membrane and beads are oppositely charged. Bead adsorption was not observed when both surfaces are evenly charged. The latter was found to be influenced by electrolyte concentration. High salt concentrations or present bivalent ions reduced the electrostatic repulsion between evenly charged surfaces and led to membrane fouling. Low salt concentrations did not influence the electrostatic repulsion. Thus, critical salt concentrations were determined and used to identify the critical zeta potential. In addition, the fouling of a zwitterionic membrane surface was investigated regarding its pH dependence. A critical zeta potential that is associated with membrane fouling was identified. The critical zeta potentials are similar for both pH and salt concentration dependence.
1. Introduction
1.1 Membrane fouling and electrostatic interactions
Membrane fouling is a problem that is encountered in nearly all membrane processes especially in aqueous media. Due to the adsorption of fouling reagents to the membrane surface pore blocking occurs. This leads to a significant loss in membrane performance.1,2So far, membrane fouling was thought to be mainly caused by hydrophobic interactions. The often used membrane polymers like polyvinylidene fluoride (PVDF) or polyethersulfone (PES) are very hydrophobic. Due to the interaction of the hydrophobic surfaces of both membrane and foulant the latter adsorbs to the membrane surface.3–11 To prevent this, membranes need to be hydrophilized. Typical methods are surface grafting reactions,4,6,7,9,11–13 electron-beam modifications,14,15 or plasma treatments.5,8,16–21 The anti-fouling property of a membrane is usually evaluated by its hydrophilicity which is determined by water contact angle measurements.22
Nevertheless, also hydrophilized membrane can foul. The reason is electrostatic interaction between the membrane and fouling reagents.23–36 These interactions are known for a long time and were already considered in the Derjaguin–Landau–Verwey–Overbeek theory.37,38 However, in membrane science the research focus shifted towards electrostatic interactions just recently. Electrostatic interactions are investigated by the charged state of a membrane surface. Determination of this charged state can be conducted by measurement of the membrane surface's zeta potential.23,28,30
In our prior studies we designed a new fouling test system to investigate electrostatic interactions undisturbed by other types of interactions.39,40 Differently charged polystyrene beads (PS, 0.2 μm) were filtered through the investigated microfiltration membranes (average pore size: PES 0.8 μm, PVDF 0.9 μm). Fouling occurred when membrane surface and beads were oppositely charged. In contrast, no bead adsorption was observed for evenly charged membranes and beads. Therefore, the electrostatic interactions are considered to be the dominant forces of this set-up. Being solely focused on electrostatic interactions is a significant advantage. This benefit is obvious especially when compared to common protein fouling tests where many different interactions overlap.40 Following the results of our previous studies, we now aim to further identify the impact of pH value and salt concentration of the surrounding media on membrane fouling.
1.2 Impact of salt concentration and pH value
When a method as described above is mainly focused on electrostatic interactions, it becomes sensitive to solution parameters like pH value, salt concentration, or ion valance. As already mentioned electrostatic interactions are determined by the surface charge of both membrane and foulant.23,41 Surface charge and zeta potential on the other hand are influenced by the electrochemical double layer.42–47 An increase in salt concentration leads to a compression of the electrochemical double layer.48 Therefore, the distance between solid surface and slipping plane (where the zeta potential is measured) is increased which directly results in a decreased zeta potential. The same is true when the pH value is changed towards the isoelectric point (IEP) of the solid. Membrane fouling that is mainly dictated by electrostatic interactions should therefore be very sensitive to changes in salt concentration or pH value of the electrolyte solution. Electrostatic repulsive forces might be weakened and the stability of the PS bead suspensions could be affected alike. The corresponding zeta potential is therefore defined as the critical zeta potential. If the zeta potential of the membrane surface decreases below this critical value fouling should occur. Nevertheless, when the zeta potential is decreased electrostatic attractive interactions might be reduced as well leading to unexpected anti-fouling. Similar investigations were carried out previously.27,41,49–52 Especially Hong and Elimelech27 did a wide ranging study on the natural organic matter fouling of nanofiltration membranes. They found that fouling increased with increasing concentration of sodium chloride. Also, the additional fouling effect of bivalent calcium ions was demonstrated at great length regarding natural organic matter.Elzo et al.41 did extensive work on the fouling of ceramic membranes using silica particles. They found that the particle adsorption depend on the pH value of the solution because the particle's zeta potential changes.
As described above, a lot of studies have already been done to show how membrane fouling is influenced by pH value, salt concentration, or ion valence. Unfortunately, the conditions (pH value, ionic strength) that are critical for membrane fouling were not completely determined, and the corresponding critical zeta potential was not identified.
Nevertheless, knowing the critical zeta potential enables to predict the fouling of a membrane. Therefore, the concept of critical zeta potential23 gives valuable insight in the process of membrane fouling and enables the prediction the latter. By determining critical zeta potential values, this paper will contribute further understanding of the conditions that are critical for membrane fouling and the adjustments which are necessary to prevent it.
Furthermore, this study is essential to fully comprehend the fouling test system introduced in our previous works.39,40 This fouling test is designed to investigate electrostatic interactions undisturbed by other types of interactions. The presented results demonstrate the impact of environmental parameters to this test system and reveal that it can still be applied when the electrolytic environment is changed. In addition, the test is applied to different membranes to demonstrate that the system is not bound to a specific membrane and can be applied to other systems as well.
2. Methods and materials
2.1 Reagents and materials
PVDF flat sheet membranes (referred to as PVDF–REF in the following) were obtained from Carl Roth (Roti–PVDF, 0.45 μm, Karlsruhe, Germany). PES flat sheet membranes were prepared using non-solvent induced phase separation (NIPS). The following chemicals were obtained from Sigma Aldrich (St. Louis, USA): aluminum oxide (Brockmann activity I, Fluka), 2,2′-azobis(2-methylpropionamidine)dihydrochloride (AIBA), calcium chloride (Fluka), lauryldimethylammonia acetate, lysine, 1-methyl-2-pyrrolidone (NMP), polystyrene sulfonate (PSS, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), potassium persulfate (KPS), sodium chloride, sodium sulfate (anhydrous, Fluka), styrene, tetraethylpentamine (TEPA).
000 g mol−1), potassium persulfate (KPS), sodium chloride, sodium sulfate (anhydrous, Fluka), styrene, tetraethylpentamine (TEPA).
2-Aminoethyl methacrylate hydrochloride (AEMA, Acros Organics), polyethylene glycol (PEG, 400 g mol−1, Acros Organics), and sodium bicarbonate were purchased from Thermo Fisher Scientific (Geel, Belgium). Other purchased chemicals: glutaraldehyde (GA, Merck, Darmstadt, Germany), n-hexane (Merck, Darmstadt, Germany), hydrochloric acid solution (0.1 M, VWR, Radnor, USA), polyethersulfone (PES, Ultrason E2010, BASF, Ludwigshafen, Germany), sodium carbonate (anhydrate, VWR, Radnor, USA), sodium hydroxide solution (0.1 M, VWR, Radnor, USA).
All chemicals are used as received. Ultrapure water was taken from a MilliQ-System (Merck Millipore, Billerica, USA). The dialysis membranes used for the bead purification were purchased from Carl Roth (cellulose acetate, Nadir, molecular weight cut-off (MWCO): 10–20 kDa, Wiesbaden, Germany).
2.2 Preparation and characterization of polystyrene beads
Suspensions of PS beads were prepared using a emulsion polymerization method. The surface charge was adjusted by the choice of initiator. First, styrene monomer was purified using an aluminum oxide column. The reaction system was composed of a three-necked flask equipped with a reflux condenser, a mechanical stirrer, and a septum. This system was filled with water, heated to 70 °C and kept under nitrogen atmosphere. Styrene was then added via syringe to get a 5 wt% emulsion. The emulsion was constantly stirred (∼200 min−1) and the reaction was started by the addition of the initiator (8 mmol L−1). KPS53 was used to generate anionic beads and AIBA54 was chosen to create a cationic bead surface. After 30 min reaction time lauryldimethylammonia acetate was added as a surfactant. The reaction was stopped after 2 h by venting the system with air. Finally, the resulting bead suspensions were washed with n-hexane and dialyzed three times using a cellulose acetate dialysis membrane.The PS bead suspensions were characterized (bead size, zeta potential, isoelectric point (IEP)) using the Malvern Zetasizer (Zetasizer Nano ZS with multipurpose titrator MPT-2, Malvern Instruments, Worcestershire, UK). Scanning electron microscopy (SEM) images (Ultra 55 SEM, Carl Zeiss Ltd, Goettingen, Germany) were taken from beads spin-coated on a silica wafer.
2.3 Membrane preparation
PES membranes were prepared using a non-solvent induced phase separation process (NIPS). A solution composed of 14 wt% PES, 65 wt% PEG and 21 wt% NMP was used. The polymer solution was casted on a glass plate with a casting knife (200 μm gap, ZWA 2121 Wasag Applicator, Zehntner Testing Instruments, Sissach, Switzerland). The polymer film was then kept in humidified air at room temperature for 5 min, followed by precipitation in cooled water (∼10 °C) for 10 min. Afterwards, the membranes were rinsed three times with water for 30 min, respectively. The pristine membranes were stored in water until further usage and will be referred to as PES–REF in the following.2.4 Membrane modification and characterization
Membrane modification was carried out using electron beam (EB) irradiation and an appropriate modification reagent. The membranes were dipped into the respective solution and the modification was performed in wet state with a custom-made electron accelerator in nitrogen atmosphere (O2 quantities < 10 ppm). The absorbed dose was adjusted by the speed of the sample transporter. Voltage and current were set to 160 kV and 10 mA, respectively.An aqueous solution of PSS (2 wt%) was used to create the PVDF–PSS and PES–PSS membranes. The EB irradiation dose was adjusted to 200 kGy.55
To generate the PVDF–TEPA and PES–TEPA membranes several reaction steps were necessary. First, EB irradiation was performed using an aqueous solution of AEMA (0.5 wt%) and a irradiation dose of 150 kGy. The membranes were rinsed with water three times for 30 min, respectively. Then, the membranes were dipped into an aqueous solution of GA (2 wt%) at pH 9.2 (NaHCO3/Na2CO3 buffer system) for 2 h. The GA solution was removed and the membranes were roughly rinsed before they were immersed into an aqueous solution of TEPA (2 wt%) at pH 9.2 for another 2 h. The reactions with GA and TEPA were repeated as described before to create a dendrimeric structure.56
The PVDF–lysine and PES–lysine membranes were prepared similar to the membranes modified with TEPA. EB irradiation was performed using a 2 wt% solution of AEMA and an irradiation dose of 200 kGy. The membranes were rinsed with water three times for 30 min, respectively. The reaction with GA was performed as described above but in the next step lysine was used instead of TEPA.39
Finally, all modified membranes were rinsed three times for 30 min, respectively.
Membrane morphology was investigated using SEM imaging (Ultra 55 SEM, Carl Zeiss Ltd., Goettingen, Germany). The samples were chromium coated (30 nm) using a Z400 sputter system (Leybold, Hanau, Germany).
Pore size distribution and porosity of the membranes were measured with a mercury porosimeter (PoreMaster 30, Quantachrome Instruments, Odelzhausen, Germany). Values of at least three different samples were averaged.
The water permeability was determined using a stainless steel pressure filter holder (16249, Sartorius, Goettingen, Germany) for dead-end filtration. A volume of at least 200 mL of deionized water was passed through the membrane (active area: 17.3 cm2) at 1 bar and the time of flow-through was recorded. Values of at least three different samples were averaged. Pure water permeation flux J was calculated following eqn (1).
 | (1) |
The chemical composition was determined using X-ray photoelectron spectroscopy (XPS, Kratos Axis Ultra, Kratos Analytical Ltd., Manchester, UK).
Surface wettability was investigated using a static water contact angle measurements system (DSA 30E, Krüss, Hamburg, Germany) and the sessile drop method. Values of at least three different samples were averaged.
Membrane zeta potentials were measured using streaming potential measurements performed with the adjustable gap cell in the SurPASS system (Anton Paar, Graz, Austria). The zeta potential ζ can be calculated based on the Smoluchowski eqn (2). Values of at least three different samples were averaged.
 | (2) |
2.5 Fouling experiments
Filtration experiments with PS beads were performed by dead-end filtration using a 50 mL stirred cell (Amicon, Merck Millipore, Billerica, USA). The membrane sample (active area: 15.9 cm2) was mounted into the stirring cell and a volume of 140 mL water was passed through the membrane at 0.1 bar to check water permeability. Afterwards, a volume of 140 mL PS bead suspension (∼50 mg L−1) was passed through the membrane under the same conditions, and filtrate fractions were taken every 20 mL. The pH and ionic strength of the filtered water and PS bead suspensions were adjusted prior to the experiment as needed. The pH of every suspension was checked prior to use with a pH electrode system (HI 3220, Hanna Instruments, Kehl, Germany). To adjust the pH value hydrochloric acid solution (0.1 M) and sodium hydroxide solution (0.1 M) were used. The ionic strength was tuned by addition of defined amounts of sodium chloride, sodium sulfate or calcium chloride. The time of flow-through was recorded for each fraction. The concentration of PS beads was monitored spectrometrically (Infinite M200, Tecan, Männedorf, Switzerland) using the UV absorption of polystyrene at 290 nm. The absorption for every starting bead suspension was checked prior to every filtration experiment. Values of at least three different samples were averaged.3. Results and discussion
The aim of this study is to show how electrolyte solution parameters influence membrane fouling. Therefore, charged polystyrene beads were filtered through a set of polymer membranes with differently charged surfaces. We already proved in prior work39,40 that fouling using this set-up mainly depends on electrostatic interactions. These interactions are determined by the surface potential of both membrane surface and fouling reagent. Therefore, they are influenced by electrolyte solution parameters like salt concentration, ion valence, or pH value.Furthermore, it should be noted that this study is focused on interactions between membrane and beads. The initial fouling is caused by interactions between membrane and beads. If beads are adsorbed to the membrane surface the membrane's pores will be blocked. The following cake layer formation and the according interactions between beads are merely consequences of this size exclusion effect. Without the initial fouling interactions between beads will also not occur. The origin of fouling can be attributed to the initial interaction between membrane and beads. Therefore, the discussion in the following will focus on interactions between membrane and beads instead of interactions between the beads itself.
The synthesis of 0.2 μm PS beads as well as the modification of polymer membranes (average pore size: PES 0.8 μm, PVDF 0.9 μm) is known from literature. Detailed information regarding the characterization of PS beads and membranes are therefore presented in the ESI.†
3.1 Impact of salt concentration and ion valence
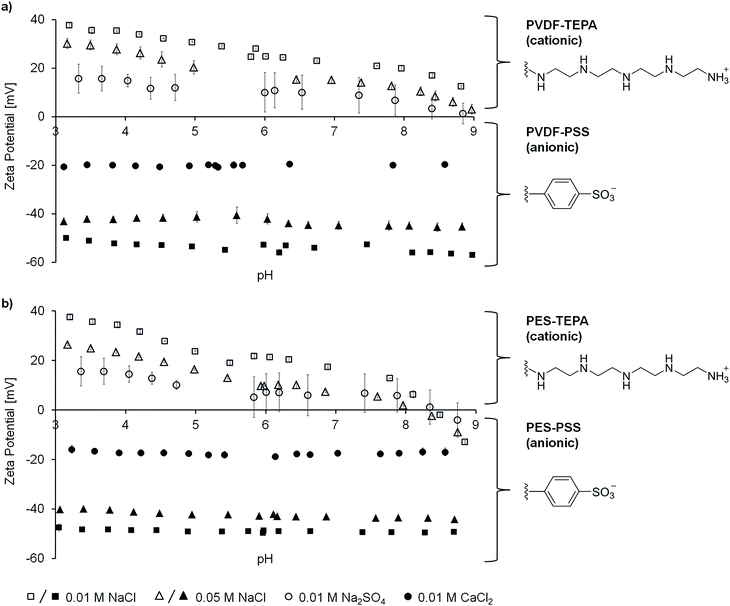
The concentration of the present ions is a crucial parameter. The fouling tendency of different membranes is highly affected by the amount of ions solved in the fouling media. To better comprehend the following fouling studies the membrane and bead surface characteristics will be briefly explained. Afterwards, the influence of salt concentration will be presented regarding electrostatic repulsive and electrostatic attractive interactions.Fig. 1a and b show the zeta potential vs. pH of PES and PVDF membranes modified with PSS (polystyrene sulfonic acid, Fig. 1) or TEPA (tetraethylpentamine, Fig. 1). The salt concentrations presented are 0.01 M and 0.05 M for sodium chloride, and 0.01 M for the respective salt containing bivalent ions. The results for other concentrations are shown in Fig. S4 in the ESI.† All error bars shown represent 95% confidence values.
 | ||
| Fig. 1 Zeta potential vs. pH at different salt concentrations and charged moieties of (a) PVDF–TEPA and –PSS membranes; (b) PES–TEPA and –PSS membranes. | ||
The zeta potential of the PVDF–TEPA membrane was determined to be +23 mV at pH 7 and 0.01 M sodium chloride. The membrane surface is highly positively charged. This can be explained by the large amount of protonated amino functions (Fig. 1) present on the membrane surface. The isoelectric point (IEP) was found at a pH value of 9.1 and does not change for the different salt concentrations because it only depends on the surface material. However, the zeta potential at pH 7 varied depending on the electrolyte solution conditions. When the sodium chloride concentration was increased to 0.05 M the PVDF–TEPA membrane's zeta potential decreased to +15 mV. This can be explained by a compression of the electrochemical double layer and the therefore shifted slipping plane. The same is true for an electrolyte solution containing 0.01 M of sodium sulfate. The bivalent sulfate ions decreased the zeta potential even stronger than the chloride ions at the same concentration.
A similar trend was found for the PVDF–PSS membrane. The sulfonic acid groups (Fig. 1) led to a highly negatively charged membrane surface. The zeta potential at pH 7 was −54 mV at a sodium chloride concentration of 0.01 M. When the concentration was increased to 0.05 M the absolute value of the zeta potential decreased to −45 mV. The same is true for calcium chloride at a concentration of 0.01 M (potential of −20 mV) due to the bivalent calcium ions. Again, these results can be explained by a compression of the electrochemical double layer. The IEP of the PVDF–PSS membrane was not recorded within the measurement range and is expected to be lower than pH 3.
Similar results were obtained for the PES membranes. The PES–TEPA membrane has a zeta potential of +18 mV, +7 mV or +6 mV at pH 7 when 0.01 M NaCl, 0.05 M NaCl, or 0.01 M Na2SO4 are used as electrolyte solutions, respectively. For the PES–PSS membrane the zeta potential at pH 7 was −49 mV, −43 mV or −17 mV at pH 7 using 0.01 M NaCl, 0.05 M NaCl, or 0.01 M CaCl2 as electrolyte solutions, respectively.
Fig. 2a shows the zeta potential vs. pH of cationic and anionic PS beads. The salt concentrations presented are 0.01 M and 0.05 M for sodium chloride, and 0.01 M for the respective salt containing bivalent ions. The results for other concentrations are shown in Fig. S2 in the ESI.† All error bars shown represent 95% confidence values. The charged moieties are presented in Fig. 2b.
 | ||
| Fig. 2 (a) Zeta potential vs. pH at different salt concentrations of charged PS beads; and (b) charged moieties of beads. | ||
It was found that cationic PS beads possess a positive zeta potential of +29 mV at pH 7 and a sodium chloride concentration of 0.01 M. The potential just slightly changes (+23 mV) when the sodium chloride concentration is raised to 0.05 M. The IEP was found at a pH value of 9. A similar trend was found for the anionic PS beads. At a sodium chloride concentration of 0.01 M and 0.05 M the zeta potential was −65 mV and −50 mV, respectively. The IEP was not recorded in the applied measurement range. The results for other concentrations are shown in Fig. S2 in the ESI.†
For both bead species a significant reduction of the absolute values of the zeta potential was found when bivalent ions were used for the electrolyte solution. The potential of cationic beads was reduced to +10 mV at pH 7 when sulfate ions were present. The presence of calcium ions led to an extenuated potential of −27 mV of the anionic beads.
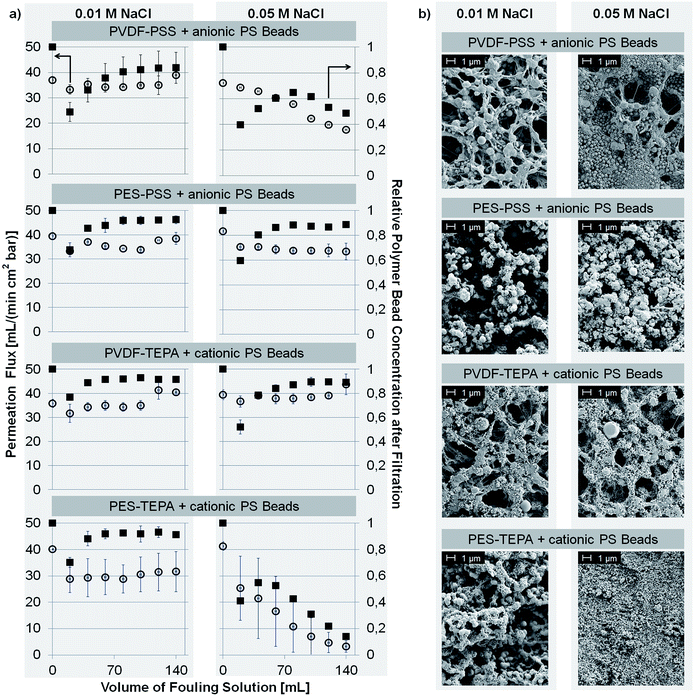
Sodium chloride concentrations of 0.01 M and 0.05 M were chosen to demonstrate how fouling depends on the salt concentration. For the filtration of anionic PS beads through the anionic PVDF–PSS membrane no fouling occurred for sodium chloride concentrations of 0.001 M (Fig. S5a in the ESI†) and 0.01 M (Fig. 3a). Neither permeation flux nor PS bead concentration declined and the structure remained predominantly open as shown in Fig. 3b. When the salt concentration was further increased fouling of the membrane occurred. Due to the decreased zeta potential (see Section 3.1.1) the electrostatic repulsive interactions were extenuated and PS beads were adsorbed to the membrane surface. The subsequent pore blocking led to a complete fouling of the membrane as shown for concentrations of sodium chloride of 0.05 M (Fig. 3) or 0.1 M (Fig. S5a†). Here, permeation flux and bead concentration decreased during the experiment and a complete pore blocking was visible in the SEM images.
Similar results were found for the fouling of the PES–PSS membrane using anionic PS beads. No flux decline or reduced concentration of PS beads was found at a sodium chloride concentration of 0.001 M (Fig. S5a†) and 0.01 M (Fig. 3a). The corresponding SEM images (Fig. S5b in the ESI† and Fig. 3b) show no pore blocking. When the salt concentration was increased to 0.05 M permeation flux and bead concentration remained unchanged. Nevertheless, incipient pore blocking was visible in the SEM image (Fig. 3a). Complete membrane fouling was observed when the sodium chloride concentration was further increased to 0.1 M (Fig. S5 in the ESI†).
Furthermore, the influence of bivalent ions was studied. The respective results are presented in Fig. S6 in the ESI.† Both PVDF–PSS and PES–PSS did not show any fouling at 0.001 M concentrated calcium chloride solution. Strong fouling occurred when the concentration was raised to 0.01 M.
The fouling of the positively charged PVDF–TEPA and PES–TEPA membranes was investigated accordingly. For both membranes electrostatic repulsion of cationic PS beads was preserved at a sodium chloride concentration of 0.001 M (Fig. S5a†) and 0.01 M (Fig. 3a). The permeation flux and the bead concentration in the filtrate remained unaffected, and no pore blocking was observed according to the SEM images. Nevertheless, when the concentration was raised to 0.05 M fouling occurred for the PES–TEPA membrane. For the PVDF–TEPA membrane only changes in the SEM images were found while permeation flux and bead concentration remained unchanged. Complete fouling for both membranes was found at a sodium chloride concentration of 0.1 M.
Then, the fouling of the PVDF–TEPA and PES–TEPA membranes towards cationic beads using sodium sulfate as electrolyte was investigated. Just like the anionic membranes the repulsive interactions remained unchanged when a 0.001 M solution of the bivalent sulfate was present. Raising the concentration to 0.01 M led to a reduced zeta potential and a less efficient repulsion of the evenly charged beads. The subsequent pore blocking and reduced permeation flux and bead concentration are presented in Fig. S6 in the ESI.†
Overall, it can be concluded that the prevention of membrane fouling due to electrostatic repulsion highly depends on the salt concentration and the valence of the ions of the electrolyte solution. At low salt concentrations the zeta potential of evenly charged membranes and beads is high enough to result in electrostatic repulsion. Nevertheless, when the concentration of sodium chloride is raised to a value of 0.05 M or higher first fouling effects can be observed. We already discussed the effect of electrolyte concentration on the corresponding zeta potential in Section 3.1.1. Combining the latter and the results of fouling experiments conducted with different electrolyte concentrations we can now define the critical zeta potential. This should be that value which is associated with membrane fouling. In our fouling experiments first fouling occurred at a sodium chloride concentration of 0.05 M. For the cationic PVDF–TEPA and PES–TEPA membranes this corresponds to a zeta potential of ∼+10 mV. This is the critical zeta potential for the repulsion between cationic surfaces. The critical zeta potential of the anionic PVDF–PSS and PES–PSS membranes was identified at a value of ∼−40 mV.
When the salt concentration is increased the zeta potential of the PS beads is decreased as well. Nevertheless, for all investigated combinations the absolute value of zeta potential of the PS beads is always higher compared to the zeta potential of the membranes. Therefore, when the absolute values of zeta potential are lowered, the zeta potential of the membranes will reach the critical value first.
When the salt concentration is increased the zeta potential of both membrane and bead is decreased. Therefore, the electrostatic attractive interactions should also decrease and become negligible at a certain critical value as shown above for the repulsive interactions. Nevertheless, fouling occurred for all conducted experiments to different degrees. The fouling was investigated using sodium chloride solutions in a range of 0.001–0.1 M. The attractive forces must be stronger than the repulsive ones. Therefore, a much higher concentrated salt solution would be necessary to successfully suppress the electrostatic attractive forces. Unfortunately, the PS bead suspensions are not stable at sodium chloride concentrations higher than 0.1 M. Therefore, it was not possible to conduct the respective experiments.
3.2 Impact of pH value
Besides the salt concentration the pH value of a solution also affects the zeta potential. To demonstrate how membrane fouling depends on the pH value the PES–lysine membrane was chosen. This membrane has a zwitterionic moiety (Fig. 4a) and the IEP is at a neutral pH value of 6.2 (Fig. 4a). Therefore, the zeta potential strongly varies over a broad range of pH (Fig. 4a). The membrane's zeta potential was found to be +40 mV at pH 4, −10 mV at pH 7, and −40 mV at pH 9 (Fig. 4a). Cationic and anionic PS beads were filtered through the membrane at pH values varying from 4 to 9. Chosen results for the trends of the permeation flux are presented in the Fig. 4b and c. A complete overview of all conducted experiments is given in Fig. S9 in the ESI.†For the filtration of cationic PS beads fouling could be prevented at pH 4. At pH 5 and pH 6 the permeation flux slowly decreased due to the reduced electrostatic repulsion between membrane and beads. For all pH values higher than 6 fouling occurred due to the missing (pH 7) or attracting (pH 8 and 9) electrostatic interactions. Compared to the assessment in Section 3.1.2 the critical zeta potential is found at pH 5 and has a value of +20 mV. This value is comparable to the critical zeta potential (+10 mV) found for the salt concentration dependence.
The adsorption of anionic beads can be prevented at pH 9 where the membrane is highly negatively charged. A medium permeation flux decline as detected for the cationic beads does not occur. The repulsive interactions at pH 8 are already too small and the critical zeta potential was ∼−30 mV. Again, this value is comparable to the critical value that was found for the salt concentration dependence (−40 mV). For all pH values below pH 8 electrostatic interactions are either too weak (pH 8–6) or attractive (pH 5 and 4).
When the pH value is varied from pH 4–9 the zeta potential of the PS beads remains unaffected in case of the anionic beads. The zeta potential of the cationic beads slightly decreases when the pH value is increased. Nevertheless, the zeta potentials of the PS beads remain stable compared to the drastic changes of the zeta potential of the PES–lysine membrane. Therefore, it can be assumed that fouling occurs because the zeta potential of the membrane falls below the critical value. An influence of the PS beads is not expected in the investigated range of pH.
4. Conclusion
The aim of this study was to investigate how environmental parameters like pH value, salt concentration, or ion valence influence the zeta potential of polymer membranes and the resulting fouling. To control electrostatic forces charged polystyrene beads were used as fouling reagents. Also, polyethersulfone and polyvinylidene fluoride membranes were modified to possess an either positive or negative surface charge. Electrostatic forces are the dominating forces for this set-up. Suspensions of beads were filtered through the membranes in different electrolytic environments. Therefore, different fouling results were observed.The conducted experiments regarding the dependence on salt concentration and ion valence revealed that:
Electrostatic repulsion between evenly charged surfaces occurs at low salt concentrations when the corresponding zeta potential's absolute values are high.
• Repulsive forces are extenuated when the salt concentration is high. Due to a compression of the electrochemical double layer the absolute value of the zeta potential is decreased. Fouling occurs when a critical zeta potential is reached.
• Electrostatic attractive interactions between oppositely charged surfaces cannot be prevented by increased salt concentrations. The remaining attractive forces are still strong enough. A zwitterionic membrane (PES–lysine, IEP at pH 6.2) was used to investigate the pH impact on fouling with PS beads. The experiments revealed that:
• Cationic beads are repelled at low pH values. Here, the membrane is positively charged and electrostatic repulsion occurs. When the pH value is increased the zeta potential decreases and fouling is observed after a critical zeta potential is reached.
• Anionic PS beads are not adsorbed to the membrane at high pH values. Here, the membrane is negatively charged and evenly charged beads are repelled. Fouling occurs when the pH value is decreased and the absolute value of the zeta potential is decreased to a critical value.
A critical zeta potential (∼−10 mV) has been theoretically predicted by Cai et al.23 for the repulsion between negatively charged membrane and foulant surfaces. Our experiments now confirm their hypothetic zeta potential value. Electrostatic interactions and the critical zeta potential need to be considered alongside the mainly investigated hydrophobic interactions. The zeta potential must be determined using the same conditions as in the desired application. If the zeta potential's absolute value is higher than the critical zeta potential, fouling should not occur. The presented bead test system can be used to investigate if a membrane is prone to fouling due to electrostatic forces. If this is the case, variations in salt concentration or pH value will reveal the critical zeta potential value.
Acknowledgements
Financial support by the Federal State of Germany and the Free State of Saxony is gratefully acknowledged.References
- C. A. Haynes and W. Norde, J. Colloid Interface Sci., 1995, 169, 313–328 CrossRef CAS
.
- M. Wahlgren and T. Arnebrant, Trends Biotechnol., 1991, 9, 201–208 CrossRef CAS PubMed
.
- I. Lundström, Phys. Scr., T, 1983, 4, 5–13 CrossRef
.
- H. Chen and G. Belfort, J. Appl. Polym. Sci., 1999, 72, 1699–1711 CrossRef CAS
.
- N. Saxena, C. Prabhavathy, S. De and S. DasGupta, Sep. Purif. Technol., 2009, 70, 160–165 CrossRef CAS
.
- B. Van der Bruggen, J. Appl. Polym. Sci., 2009, 114, 630–642 CrossRef CAS
.
- Y. Chang, C.-Y. Ko, Y.-J. Shih, D. Quémener, A. Deratani, T.-C. Wei, D.-M. Wang and J.-Y. Lai, J. Membr. Sci., 2009, 345, 160–169 CrossRef CAS
.
- P. K. Chu, J. Y. Chen, L. P. Wang and N. Huang, Mater. Sci. Eng., R, 2002, 36, 143–206 CrossRef
.
- J. R. Du, S. Peldszus, P. M. Huck and X. Feng, Water Res., 2009, 43, 4559–4568 CrossRef CAS PubMed
.
- K. C. Khulbe, C. Feng and T. Matsuura, J. Appl. Polym. Sci., 2010, 115, 855–895 CrossRef CAS
.
- S. X. Liu, J. T. Kim and S. Kim, J. Food Sci., 2008, 73, E143–E150 CrossRef CAS PubMed
.
- Q. Shi, Y. Su, W. Chen, J. Peng, L. Nie, L. Zhang and Z. Jiang, J. Membr. Sci., 2011, 366, 398–404 CrossRef CAS
.
- M. Ulbricht, Polymer, 2006, 47, 2217–2262 CrossRef CAS
.
- A. Schulze, B. Marquardt, S. Kaczmarek, R. Schubert, A. Prager and M. R. Buchmeiser, Macromol. Rapid Commun., 2010, 31, 467–472 CrossRef CAS PubMed
.
- A. Schulze, B. Marquardt, M. Went, A. Prager and M. R. Buchmeiser, Water Sci. Technol., 2012, 65, 574–580 CrossRef CAS PubMed
.
- K. Asfardjani, Y. Segui, Y. Aurelle and N. Abidine, J. Appl. Polym. Sci., 1991, 43, 271–281 CrossRef CAS
.
- A. Boulares-Pender, I. Thomas, A. Prager and A. Schulze, J. Appl. Polym. Sci., 2013, 128, 322–331 CrossRef CAS
.
- M. G. Buonomenna, L. C. Lopez, P. Favia, R. d'Agostino, A. Gordano and E. Drioli, Water Res., 2007, 41, 4309–4316 CrossRef CAS PubMed
.
- K. C. Khulbe and T. Matsuura, J. Membr. Sci., 2000, 171, 273–284 CrossRef CAS
.
- K. R. Kull, M. L. Steen and E. R. Fisher, J. Membr. Sci., 2005, 246, 203–215 CrossRef CAS
.
- N. Vandencasteele and F. Reniers, J. Electron Spectrosc. Relat. Phenom., 2010, 178–179, 394–408 CrossRef CAS
.
- T. Young, Philos. Trans. R. Soc. London, 1805, 95, 65–87 CrossRef
.
- H. Cai, H. Fan, L. Zhao, H. Hong, L. Shen, Y. He, H. Lin and J. Chen, J. Colloid Interface Sci., 2016, 465, 33–41 CrossRef CAS PubMed
.
- A. E. Childress and M. Elimelech, Environ. Sci. Technol., 2000, 34, 3710–3716 CrossRef CAS
.
- A. V. Dudchenko, J. Rolf, K. Russell, W. Duan and D. Jassby, J. Membr. Sci., 2014, 468, 1–10 CrossRef CAS
.
- M. Hadidi and A. L. Zydney, J. Membr. Sci., 2014, 452, 97–103 CrossRef CAS
.
- S. Hong and M. Elimelech, J. Membr. Sci., 1997, 132, 159–181 CrossRef CAS
.
- M. Kumar and M. Ulbricht, Polymer, 2014, 55, 354–365 CrossRef CAS
.
- K. W. Trzaskus, W. M. de Vos, A. Kemperman and K. Nijmeijer, J. Membr. Sci., 2015, 496, 174–184 CrossRef CAS
.
- K. Xiao, X. Wang, X. Huang, T. D. Waite and X. Wen, J. Membr. Sci., 2011, 373, 140–151 CrossRef CAS
.
- Q. Zhang and C. D. Vecitis, J. Membr. Sci., 2014, 459, 143–156 CrossRef CAS
.
- W. Richard Bowen, N. Hilal, M. Jain, R. W. Lovitt, A. O. Sharif and C. J. Wright, Chem. Eng. Sci., 1999, 54, 369–375 CrossRef
.
- W. R. Bowen and X. Cao, J. Membr. Sci., 1998, 140, 267–273 CrossRef CAS
.
- W. R. Bowen, A. Mongruel and P. M. Williams, Chem. Eng. Sci., 1996, 51, 4321–4333 CrossRef CAS
.
- W. R. Bowen and F. Jenner, Adv. Colloid Interface Sci., 1995, 56, 141–200 CrossRef CAS
.
- W. R. Bowen, J. I. Calvo and A. Hernández, J. Membr. Sci., 1995, 101, 153–165 CrossRef CAS
.
- B. V. Derjaguin and L. Landau, Acta Physicochim. URSS, 1941, 14, 633–662 Search PubMed
.
- E. J. W. Verwey and J. T. G. Overbeek, Theory of the Stability of Lyophobic Colloids, Elsevier Publishing Company, Inc., Amsterdam, 1948 Search PubMed
.
- D. Breite, M. Went, A. Prager and A. Schulze, Polymers, 2015, 7, 2017–2030 CrossRef CAS
.
- D. Breite, M. Went, I. Thomas, A. Prager and A. Schulze, RSC Adv., 2016, 6, 65383–65391 RSC
.
- D. Elzo, I. Huisman, E. Middelink and V. Gekas, Colloids Surf., A, 1998, 138, 145–159 CrossRef CAS
.
- D. L. Chapman, Philos. Mag., 1913, 25, 475–481 CrossRef
.
- L. G. Gouy, C. R. Acad. Sci., 1909, 149, 654–657 Search PubMed
.
- L. G. Gouy, J. Phys, 1910, 9, 457–468 Search PubMed
.
- D. C. Grahame, Chem. Rev., 1947, 41, 441–501 CrossRef CAS PubMed
.
- O. Stern, Z. Elektrochem., 1924, 30, 508 CAS
.
- M. V. Smoluchowski, Bull. Int. Acad. Sci. Cracovie, 1903, 3, 184–199 Search PubMed
.
- R. M. Pashley, J. Colloid Interface Sci., 1981, 80, 153–162 CrossRef CAS
.
- C. Werner, H. Körber, R. Zimmermann, S. Dukhin and H.-J. Jacobasch, J. Colloid Interface Sci., 1998, 208, 329–346 CrossRef CAS PubMed
.
- R. Zimmermann, S. Dukhin and C. Werner, J. Phys. Chem. B, 2001, 105, 8544–8549 CrossRef CAS
.
- D.-J. Chang, F.-C. Hsu and S.-J. Hwang, J. Membr. Sci., 1995, 98, 97–106 CrossRef CAS
.
- W. Norde and J. Lyklema, J. Colloid Interface Sci., 1978, 66, 277–284 CrossRef CAS
.
- S. Inukai, T. Tanma, S. Orihara and M. Konno, Chem. Eng. Res. Des., 2001, 79, 901–905 CrossRef CAS
.
- D. Duracher, F. Sauzedde, A. Elaissari, A. Perrin and C. Pichot, Colloid Polym. Sci., 1998, 276, 219–231 CAS
.
- A. Schulze, M. F. Maitz, R. Zimmermann, B. Marquardt, M. Fischer, C. Werner, M. Went and I. Thomas, RSC Adv., 2013, 3, 22518–22526 RSC
.
- A. Boulares-Pender, A. Prager, C. Elsner and M. R. Buchmeiser, J. Appl. Polym. Sci., 2009, 112, 2701–2709 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra19239d |
| This journal is © The Royal Society of Chemistry 2016 |