Evidence of competition in the incorporation of Co2+ and Mn2+ ions into the structure of ZnTe nanocrystals
Abstract
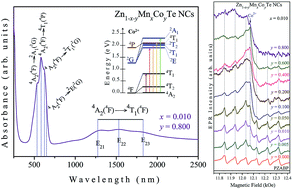
Glass-embedded Zn1−x−yMnxCoyTe nanocrystals (with x = 0.01 and y varying from 0.000 to 0.800) were successfully synthesized using a protocol based on the melting–nucleation synthesis route and herein investigated by several experimental techniques. Optical absorption (OA) provides evidence of the incorporation of substitutional Co2+ ions in the semiconducting Zn1−x−yMnxCoyTe lattice and shows that with increasing Co concentration these ions may also be dispersed into the glass matrix, causing structural disorder. Atomic force microscopy (AFM) images reveal the size of the nanocrystals and magnetic force microscopy (MFM) gives evidence of the paramagnetic behavior of the doped samples. X-ray diffraction (XRD) and Raman scattering reveal that high Co concentrations increase the structural disorder in the host glass matrix. Electron paramagnetic resonance (EPR) spectroscopy reveals the presence of Mn2+ ions inside and at (or near) the surface of the ZnTe nanocrystals, and also that the increase of Co doping concentration reduces the incorporation of Mn2+ ions into the ZnTe structure, simultaneously increasing their dispersion into the glass matrix.

 Please wait while we load your content...
Please wait while we load your content...