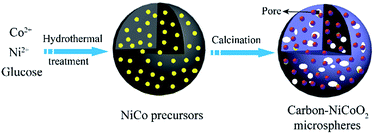
Facile synthesis of carbon–NiCoO2 composite microspheres with pitaya-type structure and their application in supercapacitors†
Yongchao Maa,
Ping Liua,
Qingzhi Liua,
Zhiwei Chenb,
Dan Qua and
Jinsheng Shi*a
aQingdao Agricultural University, Qingdao 266109, People's Republic of China. E-mail: jsshiqn@aliyun.com; Fax: +86-532-86080213; Tel: +86-532-88030161
bSchool of Life Sciences, Shandong University of Technology, Zibo 255049, People's Republic of China
First published on 1st August 2016
Abstract
Three-dimensionally porous oxide composite materials have recently attracted increasing interest because of their exciting potential in electrochemical energy conversion and storage. Herein, novel carbon–NiCoO2 microsphere composites with a pitaya-type structure have been prepared for the first time via a one-pot hydrothermal synthesis followed by annealing treatment. These composite microspheres are composed of NiCoO2 nanoparticles inserted into porous carbon microspheres. Furthermore, the prepared carbon–NiCoO2 electrodes exhibit excellent rate capacity when used as supercapacitor electrodes. Under a current density of 1 and 10 A g−1, the optimized composite electrode exhibits specific capacitance of 507 and 390 F g−1, respectively. The excellent electrochemical properties can possibly be attributed to the intrinsic nature of NiCoO2, the ultrafine NiCoO2 particles, and sufficient space being available to interact with the electrolyte. This facile synthesis method may be extended to prepare other carbon-based metal oxides, which may find application in catalysts and adsorption due to their unique structural features.
1. Introduction
Supercapacitors, as the most important next-generation energy storage devices, have attracted considerable attention because of their high power density and long lifespan, fast charge and discharge rates and low maintenance cost.1–5 Various materials were used as capacitive materials, such as carbon nanotubes, graphene, metal composites and polymers.6–9 Recently, transition metal oxides have become one of the most promising materials for supercapacitor electrodes due to their good stability and multiple oxidation states for electron-transfer processes.10–12 Among them, RuO2, MnO2, NiO and Co3O4 have been the most researched.13–15 However, these transition metal oxides suffer from various problems, such as low electronic conductivity, high cost, toxicity and poor cycling stability.16,17Compared with mono-oxides, mixed transition metal oxides combine the advantages of different metal cations and possess multiple oxidation states, leading to higher electrical conductivity and better capacitance.18–20 One of the most promising materials based on transition metal oxide is the mixed Ni and Co oxide, which is a stable and cheap electrode material.21 The performance of transition metal oxides is highly dependent on the micro-nanostructures of the materials in various applications.22–25 Therefore, synthesis of materials with a rational designed morphology provides one of the most practicable methods to create high-performance supercapacitors. To date, there have been several reports on the synthesis and electrochemical evaluation of NiCo2O4 material with various morphologies.26–28 Furthermore, a few of NiCoO2 materials, as another mixed Ni and Co oxide, have been reported on the synthesis and electrochemical evaluation. Ding et al. reported the synthesis of hierarchical NiCoO2 nanosheets nanotubes by a mild solution method.16 Nitrogen doped reduced graphene sheet–NiCoO2 composite was prepared by a simple one-step hydrothermal synthesis.17 However, the study on the synthesis of carbon–NiCoO2 composite microsphere with pitaya-type structure has been rarely been reported.
Herein, carbon–NiCoO2 spheres with pitaya-type structure have been successfully prepared via a simple and environmental approach involving the one-pot hydrothermal synthesis and calcinations for the first time. The structurally unique composites are composed of NiCoO2 nanoparticles incorporated into porous carbon spheres. The as-obtained novel pitaya-type composites with high surface area (657.4 m2 g−1) manifest excellent electrochemical properties, particularly the rate capability and cycling stability. The superior electrode chemical performances of the composites are ascribed to their high specific surface areas, mesoporosity and superior electronic conductivity. Finally, the preparation concepts are expected to be useful for synthesis of other metal oxides widely used in a variety of application such as adsorption, catalysis, drug delivery, and especially supercapacitor applications.
2. Experimental
2.1 Synthesis of carbon–NiCoO2 composite microspheres
In a typical experiments, 20.1 mmol of glucose and the desired amount of Co(NO3)2·6H2O and Ni(NO3)2·6 H2O were added to 60 mL of distilled water under vigorous stirring to form a homogeneous solution. After being stirred for 30 min, the resultant mixture was placed in a 100 mL Teflon-lined stainless steel autoclave and maintained at 180 °C for 24 h. The black products were then collected, washed three times with water and ethanol. The prepared samples were dried at 80 °C for 6 h, which is denoted as NiCo-precursors. To obtain the carbon–NiCoO2 microspheres, the as-prepared NiCo-precursor was calcinated at 600 °C for 2 h.2.2 Characterization
The crystal structures of the as-obtained products were recorded by X-ray diffraction (XRD) with a Bruker D8 diffractometer (Cu Kα radiation, λ = 0.15406 nm). The morphology of the as-prepared samples was examined with scanning electron microscope (SEM, S-4800, Hitachi). The transmission electron microscopic (TEM) images were acquired with a JOEL JEM 2001 microscope. Surface analysis of the studied samples was performed using XPS (VGESCA-LABMKIIX-ray photoelectronic spectrometer). Thermogravimetric analysis (TGA-2050 (TA Corp.)) was also conducted to determine the composition of samples. The TGA measurements were carried out at a heating rate of 10 °C min−1 from 20 to 800 °C with an air flow-rate of 100 mL min−1. The measurements of the specific surface area and the analysis of the porosity of carbon–NiCoO2 products were performed through measuring N2 adsorption–desorption isotherms at 77 K, using a Quantachrome NOVA 2200e system.2.3 Electrochemical measurements
The conclusions section should come in this section at the end of the article, before the acknowledgements. For the electrochemical measurements, the as-prepared sample was mixed with acetylene black as the conducting material and polytetrafluoroethylene (PTFE) binder, in a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. A small amount of distill water was then added to make more homogeneous mixture. Then, the slurries were coated on the nickel foam substrates (1.0 cm × 1.0 cm). After being dried at 80 °C for 2 h, the as-formed electrodes loaded with the active material were then pressed at 14 MPa. Before and after the sample was dried thoroughly, the nickel foam was weighed. The obtained NiCo2O4 modified electrode was then used as a working electrode. A platinum plate (1 cm2) and saturated calomel electrode (SCE) were used as a counter electrode and reference electrode, respectively. The electrolyte was a 3 M KOH aqueous solution.
10. A small amount of distill water was then added to make more homogeneous mixture. Then, the slurries were coated on the nickel foam substrates (1.0 cm × 1.0 cm). After being dried at 80 °C for 2 h, the as-formed electrodes loaded with the active material were then pressed at 14 MPa. Before and after the sample was dried thoroughly, the nickel foam was weighed. The obtained NiCo2O4 modified electrode was then used as a working electrode. A platinum plate (1 cm2) and saturated calomel electrode (SCE) were used as a counter electrode and reference electrode, respectively. The electrolyte was a 3 M KOH aqueous solution.
The electrochemical performance of these electrodes was evaluated using cyclic voltammetry (CV), galvanostatic charge–discharge and electrochemical impedance spectroscopy (EIS) measurements. The electrochemical measurements were conducted in a three compartment cell using a LAND battery test instrument (CT2001A, Wuhan, China). Cyclic voltammetry (CV) was conducted by LK2005A electrochemical workstation with voltage scan rates of 2 mV s−1, 5 mV s−1, 10 mV s−1, 20 mV s−1 and 30 mV s−1. The galvanostatic charge–discharge (CD) tests were conducted at the currents of 1 A g−1, 2 A g−1, 4 A g−1, 8 A g−1 and 10 A g−1. The electrochemical impedance spectroscopy (EIS) measurements were carried out by applying an AC voltage with 5 mV amplitude in a frequency range from 0.01 Hz to 100 kHz at open circuit potential.
The specific capacitances were calculated from GCD curves according to the following equations:
3. Results and discussion
In this study, carbon spheres were employed as the sacrificial templates to fabricate the carbon–NiCoO2 product. The thermal decomposition of as-prepared NiCo precursor was investigated by TG to assess the following calcination procedure, as shown in Fig. S1.† The purity and crystallinity of the as-obtained products were investigated by XRD. For the NiCo precursors, a broad diffraction peak between 10 and 30 ° is assign to crystal faces of graphite-like structure.30 After calcination, the diffraction pattern of carbon–NiCoO2 composites can be indexed to spinel NiCoO2 (JCPDS no. 10-0118). The broad and weak peaks of NiCoO2 suggest its small crystalline size (Fig. 1). | ||
| Fig. 1 XRD patterns of (a) NiCo precursor spheres and (b) pitaya-type carbon–NiCoO2 composite spheres. | ||
The Raman spectra of the prepared NiCo precursor and final samples were shown in Fig. 2. In the Raman spectrum of the NiCo precursors, two prominent peaks at 1374 and 1591 cm−1 could be observed. The peak at 1591 cm−1 (G-band) is attributed to the vibration of sp2 hybridized carbon atoms in a 2D hexagonal lattice, while the peak at 1374 cm−1 (D-band) is associated with the vibrations of carbon atoms with dangling bonds in plane terminations of disordered graphite from the defects and disorders of structures in carbon materials.31 The intensity ratio of D-band to G-band (ID/IG) depends on the type of graphitic materials and reflects the graphitization degree. The valves of ID/IG for the NiCo precursor and carbon–NiCoO2 composite microspheres samples are 0.71 and 0.72, respectively. The result suggests that the calcination at 600 °C cannot decrease the graphitization degree.
The morphology and microstructure of the samples were investigated by SEM, TEM, HRTEM and selected area electron diffraction (SAED). Fig. 3a shows the typical SEM images of the carbon spheres prepared via the hydrothermal treatment of glucose/water solutions without metal ions. Fine monodisperse spheres with average diameter of 300 nm are clearly visible. As shown in Fig. 3b and c, the as-prepared carbon–NiCoO2 precursors exhibit regular spherical shape with diameter of about 4 μm, which are larger than those of glucose-based carbon spheres due to the presence of metal ions. After calcinations of the precursors at 600 °C for 2 h, the obtained NiCoO2 metal oxide product still remains the spherical morphology, although the internal nanostructures may have been changed by opening pore channels due to the expulsion of CO2 and H2O gas during thermal decomposition process (Fig. 3d–f). Compared with the surface structure (Fig. 3c) of the precursors, a relatively rougher surface in calcinated products was observed, indicating that the spheres are composed of small primary particles. Furthermore, the average diameter of the corresponding NiCoO2 metal oxides decreases, which is possibly ascribed to the collapse of part of the internal 3D porous structures during the thermal decomposition process.
 | ||
| Fig. 3 SEM images of (a) carbon spheres, (b, c) NiCo-precursor and (d–f) carbon–NiCoO2 microspheres. | ||
The detailed morphological and crystallographic properties of the as-obtained NiCo precursors and carbon–NiCoO2 microspheres were identified by TEM, HRTEM and SAED. As shown in Fig. 3a and b, the precursor sphere is solid. Typical TEM image of the sample (Fig. 3c and d) clearly depicts the pitaya-type structure composed of NiCoO2 nanoparticles inserted into the carbon spheres. There are many pores within the carbon microspheres, which can be attributed to the expulsion of CO2 and H2O gas during thermal decomposition process. This observation is consistent with the SEM analysis (Fig. 3f). The magnified TEM image (Fig. 4d) shows that a transparent feature on their edges are decorated with porous structures on their surface, which infers that it could provide high specific area. The HRTEM image (Fig. 4e) of the remarked area with randomly orientated lattice phase reveals the polycrystalline characteristic of the as-obtained products. In addition, in the HRTEM image, the clearly resolved lattice fringes can be discerned and the lattice spacing is calculated to be about 0.24, and 0.21 nm corresponding to the (111) and (200) planes of NiCoO2, respectively. The SAED pattern (Fig. 4f) displays well-defined rings, which further confirms that the samples are of polycrystalline nature, which is identical with the aforementioned result from the HRTEM.
 | ||
| Fig. 4 TEM images of the as-prepared (a, b) NiCo-precursor and (c–e) pitaya-type carbon–NiCoO2 microspheres. (f) The SAED patterns of the carbon–NiCoO2 microspheres. | ||
The formation mechanism (Fig. 5) of the carbon–NiCoO2 microspheres with pitaya-type structure can be depicted as two-step process. First, during the hydrothermal process, the carbohydrate used as a carbon precursor is subjected to dehydration, condensation, polymerization and aromatization, and finally carbon spheres are formed.32 Meanwhile, an amount of Ni2+ and Co2+ ions are wrapped into the carbon spheres. On the other hand, the metal ions can also attach to the surface of the aforementioned carbon spheres, which is due to a considerable amount of reactive oxygen-containing group on the surface of carbon spheres.33 The subsequent calcination treatment leads to the formation of carbon–NiCoO2 microspheres with the pitaya-type structures.
The specific surface area, average pore size and mesoporous volume of materials play significant roles in enhancing electrochemical performance. With insight into the porosity of the as-prepared NiCoO2, the nitrogen adsorption–desorption measurements were performed, as shown in Fig. 6. The shape of the isotherms is type IV, indicating the existence of mesopores in the composite.34 According to the investigative results, it is clear that the as-prepared 3D microsphere possesses a high specific area of 657.4 m2 g−1. The corresponding pore size distribution of the samples was shown in Fig. S3b.† Agreeing with the TEM results, the composite shows a pore distribution from 5 to 100 nm, which could be mainly contributed by the space between particles. The high specific surface area and porous structure of the carbon–NiCoO2 microspheres would be beneficial for the increase of the electrolyte/electrode contact area and provide sufficient active sites for Faraday reaction.
 | ||
| Fig. 6 (a) Nitrogen adsorption–desorption isotherm and (b) the corresponding pore size distribution curves of the pitaya-type carbon–NiCoO2 spheres. | ||
The large surface area and good electrical conductivity will endow the composite electrodes with promising electrochemical performance for supercapacitors. To explore the potential application of the of carbon–NiCoO2 microspheres, the sample was fabricated into a supercapacitor electrode and the capacitive performance was evaluated by cyclic voltammograms (CV) and galvanostatic charge–discharge (GCD) measurements. Fig. 7a shows the typical CV curves of carbon–NiCoO2 electrode material in a 3 M KOH aqueous electrolyte at various scan rates ranging from 2 to 30 mV s−1. A pair of obvious redox peaks appears within the potential ranging from 0 to 0.41 V for all sweep rates, which indicates that the NiCoO2 can work efficiently as pseudocapacitor electrodes. This pair of peaks is mainly due to M–O/M–O–OOH (M = Co, Ni). The high-powder characteristic of the NiCoO2 electrode can be testified from the voltammetric response at different scan rates. All the curves show a similar shape, and the current density increases with the increasing scan rate. In addition, with the increasing scan rate, the reduction peaks shift to a more negative position, while oxidation peaks barely shift to a more positive position, which indicates that the internal diffusion resistance basically maintains the same level with the increasing scan rate.17
Fig. 7b presents the GCD curves of the carbon–NiCoO2 at various current densities from 1 to 10 A g−1. The voltage plateau of the electrode is in accordance with the peaks observed in the CV curves. The internal resistance drop mainly results from the current of the bulk electrode.35 The average specific capacitance of NiCoO2 can be calculated from the galvanostatic charge–discharge curves, as shown in Fig. 7c. The NiCoO2 possesses specific capacitances of 507.3, 468.3, 429.3, 409.8 and 390.2 F g−1 at the discharge currents of 1, 2, 4, 8 and 10 A g−1, respectively. Due to the decreasing utilization of active materials caused by the increased diffusion of ions, the specific capacitance decreases with the increasing current density.36
The cycle stability of carbon–NiCoO2 composite is demonstrated in Fig. 7d. After the first 2000 cycles, the specific capacitance of the sample decreases. Initially, the NiCoO2 composites are completely activated through the intercalation and de-intercalation of electrolyte ions and lead to the increase of active sites of the electrodes, thus improving the specific capacitance.37,38 After a 3000 cycle test, the specific capacitance is maintained at 97% of the total specific capacitance. The integrated galvanostatic charge–discharge curves at a current density of 1 A g−1 from 775![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 974 to 778
974 to 778![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 469 s, shown in the inset of Fig. 7d, indicate that the columbic efficiency is nearly 100% during this period. The outstanding cycling stability of NiCoO2 composite electrode can be attributed to the excellent connection between NiCoO2 and carbon materials. NiCoO2 nanoparticles standing intimately in carbon spheres lead to barrier-free contact, which decreases the internal resistance effectively.
469 s, shown in the inset of Fig. 7d, indicate that the columbic efficiency is nearly 100% during this period. The outstanding cycling stability of NiCoO2 composite electrode can be attributed to the excellent connection between NiCoO2 and carbon materials. NiCoO2 nanoparticles standing intimately in carbon spheres lead to barrier-free contact, which decreases the internal resistance effectively.
EIS tests were carried out to further understand the electrochemical behavior of the NiCoO2 composite electrode after 3000 cycles. The Nyquist plots of the electrode before and after 3000 cycles are shown Fig. 8. It can be observed that the carbon–NiCoO2 electrode exhibits a small real axis intercept and negligibly depressed semicircle in the high frequency range, indicating small active bulk material resistance and a low interfacial resistance between the current collector and the active material, electrolyte resistance as well as low charge transfer resistance.39,40 Specially, in the high frequency area, the solution resistance (Rs) after 3000 cycles decreased from 0.74 to 0.66 Ω at the high-frequency intercept of the real axis, indicating the electron conductivity of the carbon–NiCoO2 electrode represents an enhanced tendency. The linear part corresponds to the Warburg impedance (W), which is described as diffusive impendence of the OH− ion within the electrode. For an ideal supercapacitor, the Nyqusit plot should be a vertical line at low frequency, which is parallel to the imaginary impedance axis.17 After 3000 cycles, the steeper line is much closer to the ideal behavior and demonstrates a faster kinetics of diffusion process.
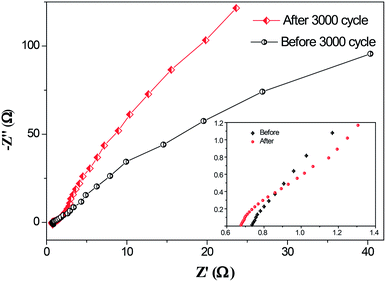 | ||
| Fig. 8 EIS curves of carbon–NiCoO2 composite electrode before and after 3000 cycles by GCD test, while an enlargement of the high frequency region is shown in the inset. | ||
4. Conclusions
In summary, we showed a facile process for the synthesis of uniform carbon–NiCoO2 microspheres with pitaya-type structure by a one-pot hydrothermal route combined with annealing treatment. Instead of fabrication by a multistep process that involves the formation of the carbon spheres using hydrothermal treatment, isolation and purification of the carbon spheres, coating of the sacrificial cores with the metal oxide precursors, and subsequent removal of the carbon cores via calcinations, we add metal salt directly to the carbohydrate solutions in water, followed by a hydrothermal treatment. Upon calcination, carbon–NiCoO2 composite microspheres were obtained. The approach has been proved to be a green route without using an organic template and any etching process, where the corrosive acid or base are usually used as the etching agents. In particular, the method is suitable for high-yield mass production of NiCoO2 with controllable properties, based on its simple and environmentally friendly preparation process. A possible formation mechanism was also proposed as well. When evaluated as the electrode material in a three-electrode system, the composites demonstrated excellent electrochemical performances with a high specific capacitance of 507 at 1 A g−1, remarkable capacity retention rate of 77% at 10 A g−1 and exceptional cycling stability with 98.5% of its initial capacity retention over 3000 cycles at 1 A g−1.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31071538), Natural Key Science Foundation of Shandong Province (ZR2013FB001), the Research Fund for Technology Upgrading of Large Scientific Instruments and Equipment in Shandong Province (2013SJGZ01) and Science and Technology Development Plan of Shandong Province, China (2014GNC110013).Notes and references
- F. Li, J. Chen, X. Wang, M. Xue and G. F. Chen, Adv. Funct. Mater., 2015, 25, 4562 CrossRef.
- Y. Zhu, Z. Wu, M. Jing, H. Hou, Y. Yang, Y. Zhang, X. Yang, W. Song, X. Jia and X. Ji, J. Mater. Chem. A, 2015, 3, 866–877 CAS.
- X. Cao, B. Zheng, W. Shi, J. Yang, Z. Fan, Z. Luo, X. Rui, B. Chen, Q. Yan and H. Zhang, Adv. Mater., 2015, 27, 4695–4701 CrossRef CAS PubMed.
- Z. Yu, L. Tetard, L. Zhai and J. Thomas, Energy Environ. Sci., 2015, 8, 702–730 CAS.
- T. Zhu, E. R. Koo and G. W. Ho, RSC Adv., 2015, 5, 1697–1704 RSC.
- H. Wang, Y. Wang, Z. Hu and X. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 6827–6834 CAS.
- N. Kurra, M. K. Hota and H. N. Alshareef, Nano Energy, 2015, 13, 500–508 CrossRef CAS.
- S. Wang, L. Gai, J. Zhou, H. Jiang, Y. Sun and H. Zhang, J. Phys. Chem. C, 2015, 119, 3881–3891 CAS.
- X. Zheng, Z. Gu, Q. Hu, B. Geng and X. Zhang, RSC Adv., 2015, 5, 17007–17013 RSC.
- F. Yang, Q. Xie, H. Zhang, S. Yu, X. Zhang and Y. Shen, Sens. Actuators, B, 2015, 210, 232–240 CrossRef CAS.
- Y. Huang, Y.-E. Miao, H. Lu and T. Liu, Chem.–Eur. J., 2015, 21, 10100–10108 CrossRef CAS PubMed.
- G. Anandha Babu, G. Ravi, T. Mahalingam, M. Kumaresavanji and Y. Hayakawa, Dalton Trans., 2015, 44, 4485–4497 RSC.
- S.-C. Hong, S. Kim, W.-J. Jang, T.-H. Han, J.-P. Hong, J.-S. Oh, T. Hwang, Y. Lee, J.-H. Lee and J.-D. Nam, RSC Adv., 2014, 4, 48276–48284 RSC.
- S.-I. Kim, J.-S. Lee, H.-J. Ahn, H.-K. Song and J.-H. Jang, ACS Appl. Mater. Interfaces, 2013, 5, 1596–1603 CAS.
- X. Xie, C. Zhang, M.-B. Wu, Y. Tao, W. Lv and Q.-H. Yang, Chem. Commun., 2013, 49, 11092–11094 RSC.
- X. Xu, H. Zhou, S. Ding, J. Li, B. Li and D. Yu, J. Power Sources, 2014, 267, 641–647 CrossRef CAS.
- Y. Xu, J. Wei, L. Tan, J. Yu and Y. Chen, J. Mater. Chem. A, 2015, 3, 7121–7131 CAS.
- Q. Wang, L. Zhu, L. Sun, Y. Liu and L. Jiao, J. Mater. Chem. A, 2015, 3, 982–985 CAS.
- W. Li, K. Xu, G. Song, X. Zhou, R. Zou, J. Yang, Z. Chen and J. Hu, CrystEngComm, 2014, 16, 2335–2339 RSC.
- Y. Xiao, Y. Lei, B. Zheng, L. Gu, Y. Wang and D. Xiao, RSC Adv., 2015, 5, 21604–21613 RSC.
- D. Kong, W. Ren, C. Cheng, Y. Wang, Z. Huang and H. Y. Yang, ACS Appl. Mater. Interfaces, 2015, 7, 21334–21346 CAS.
- C. X. Guo, G. Yilmaz, S. Chen, S. Chen and X. Lu, Nano Energy, 2015, 12, 76–87 CrossRef CAS.
- L. Mi, W. Wei, S. Huang, S. Cui, W. Zhang, H. Hou and W. Chen, J. Mater. Chem. A, 2015, 3, 20973–20982 CAS.
- L. Gu, Y. Wang, R. Lu, W. Wang, X. Peng and J. Sha, J. Power Sources, 2015, 273, 479–485 CrossRef CAS.
- L.-F. Chen, Z.-Y. Yu, J.-J. Wang, Q.-X. Li, Z.-Q. Tan, Y.-W. Zhu and S.-H. Yu, Nano Energy, 2015, 11, 119–128 CrossRef CAS.
- Y. Lei, J. Li, Y. Wang, L. Gu, Y. Chang, H. Yuan and D. Xiao, ACS Appl. Mater. Interfaces, 2014, 6, 1773–1780 CAS.
- F. Deng, L. Yu, G. Cheng, T. Lin, M. Sun, F. Ye and Y. Li, J. Power Sources, 2014, 251, 202–207 CrossRef CAS.
- J. Zhu, Z. Xu and B. Lu, Nano Energy, 2014, 7, 114–123 CrossRef CAS.
- F. Deng, L. Yu, M. Sun, T. Lin, G. Cheng, B. Lan and F. Ye, Electrochim. Acta, 2014, 133, 382–390 CrossRef CAS.
- W. Zhao, Y. Wang, Y. Yang, J. Tang and Y. Yang, Appl. Catal., B, 2012, 115, 90–99 CrossRef.
- Y. Fan, P.-F. Liu, Z.-J. Yang, T.-W. Jiang, K.-L. Yao, R. Han, X.-X. Huo and Y.-Y. Xiong, Electrochim. Acta, 2015, 163, 140–148 CrossRef CAS.
- X. Sun and Y. Li, Angew. Chem., Int. Ed., 2004, 43, 597–601 CrossRef PubMed.
- C. Cheng, Y. Huang, N. Wang, T. Jiang, S. Hu, B. Zheng, H. Yuan and D. Xiao, ACS Appl. Mater. Interfaces, 2015, 7, 9526–9533 CAS.
- H. Xia, C. Hong, B. Li, B. Zhao, Z. Lin, M. Zheng, S. V. Savilov and S. M. Aldoshin, Adv. Funct. Mater., 2015, 25, 627–635 CrossRef CAS.
- J. Kang, A. Hirata, H. J. Qiu, L. Chen, X. Ge, T. Fujita and M. Chen, Adv. Mater., 2014, 26, 269–272 CrossRef CAS PubMed.
- G. Zhang, W. Li, K. Xie, F. Yu and H. Huang, Adv. Funct. Mater., 2013, 23, 3675–3681 CrossRef CAS.
- Z. Zhu, J. Ping, X. Huang, J. Hu, Q. Chen, X. Ji and C. E. Banks, J. Mater. Sci., 2012, 47, 503–507 CrossRef CAS.
- J. Pu, J. Wang, X. Jin, F. Cui, E. Sheng and Z. Wang, Electrochim. Acta, 2013, 106, 226–234 CrossRef CAS.
- Y. Ma, H. Jiang, Q. Liu, W. Kang and J. Shi, New J. Chem., 2015, 39, 7495–7502 RSC.
- Y. Zhu, X. Pu, W. Song, Z. Wu, Z. Zhou, X. He, F. Lu, M. Jing, B. Tang and X. Ji, J. Alloys Compd., 2014, 617, 988–993 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra19113d |
| This journal is © The Royal Society of Chemistry 2016 |