Stable yet reactive cationic gold catalysts with carbon based counterions†
Abstract
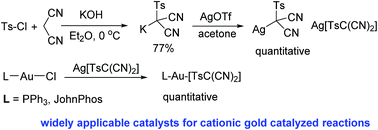
L–Au–[TsC(CN)2] are new cationic gold catalysts with a carbon based counterion, which are widely applicable for gold catalyzed reactions. For reactions which need highly reactive gold catalysts, a Lewis acid co-catalyst can be added to increase the reactivity of L–Au–[TsC(CN)2]. L–Au–[TsC(CN)2] also have advantages of easy reaction steps and high functional group tolerance.

 Please wait while we load your content...
Please wait while we load your content...