NiFe-layered double hydroxides: a bifunctional O2 electrode catalyst for non-aqueous Li–O2 batteries
Abstract
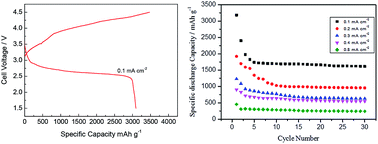
Ascertaining an appropriate cathode material for the Li–O2 battery system is one of the main tasks at present. To deal with this, in this study, a highly active and stable NiFe-layered double hydroxide (LDH) is synthesized via a hydrothermal method. The characterisation results showed the presence of LDH nanoplates covered with Ni and Fe nanoparticles. In addition to the as prepared NiFe-LDH, its calcinated products at three different temperatures, 300, 500 and 800 °C are also studied for the oxygen reduction reaction (ORR) in a non-aqueous electrolyte by using cyclic voltammetry and rotating disc electrode (RDE) techniques. The as prepared NiFe-LDH exhibits a comparatively greater catalytic activity for ORR than its calcinated products. Li–O2 battery tests are then carried out to further evaluate the catalytic activity of the products in 1.0 M LiPF6 in tetraethylene glycol dimethyl ether (TEGDME). NiFe-LDH exhibited superior catalytic performance with a specific discharge capacity of ∼3218 mA h g−1 at 0.1 mA cm−2. The discharge plateau appears at 2.75 V which is close to theoretical potential (2.9–3.1 V) for Li2O2 formation. A stable specific discharge capacity of ∼1728 mA h g−1 is obtained even after 30 cycles at 0.1 mA cm−2. The discharge–recharge voltage gap is about ∼0.9 V.

 Please wait while we load your content...
Please wait while we load your content...