Nonlinear refraction and absorption activity of dimethylaminostyryl substituted BODIPY dyes†
Abstract
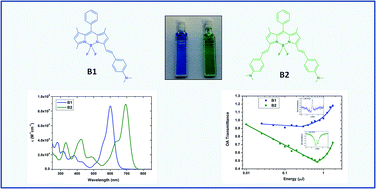
Optical and nonlinear optical properties of difluoroboradiazaindacene (BODIPY) models with attached dimethylaminostyryl substituents were studied. The optical absorption and fluorescence emission of BODIPY compounds were found to be dependent on the number of dimethylaminostyryl substituents. In order to investigate the nonlinear refraction and absorption activity, the Z-scan technique was used employing a laser generating a wavelength at 532 nm with 30 ps pulse duration. The studied dimethylaminostyryl containing BODIPY compounds showed considerable nonlinear refraction and reverse saturable absorption. The BODIPY containing a pair of dimethylaminostyryl substituents demonstrated an increased third order nonlinear optical response mostly due to the extension of its conjugated length.

 Please wait while we load your content...
Please wait while we load your content...