Development of low-temperature desulfurization performance of a MnO2/AC composite for a combined SO2 trap for diesel exhaust
X. C. Liuab,
Y. Osakac,
H. Y. Huang*a,
Huhetaolia,
J. Lid,
X. X. Yanga,
S. J. Liab and
N. Kobayashid
aKey Laboratory of Renewable Energy, Guangzhou Institute of Energy Conversion, Chinese Academy of Science, No. 2 Nengyuan Rd. Wushan, Tianhe District, Guangzhou 510640, PR China. E-mail: huanghy@ms.giec.ac.cn; Fax: +86-20-37210762; Tel: +86-20-37210762
bUniversity of Chinese Academy of Sciences, Beijing 100049, PR China
cKanazawa University, Kakuma, Kanazawa, Ishikawa 920-1192, Japan
dNagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8603, Japan
First published on 30th September 2016
Abstract
Growing concern about the removal of sulfur dioxide (SO2) from combustion exhaust has resulted in the development of desulfurization materials for a SO2 trap. In this study, a manganese dioxide/activated carbon (MnO2/AC) composite was proposed as a low temperature desulfurization material for a combined SO2 trap. The MnO2/AC composite was synthesized using a redox deposition method and characterized using scanning electron microscopy (SEM), nitrogen adsorption, X-ray fluorescence spectrometry (XRF) and Fourier transform infrared (FTIR) spectroscopy. The SO2 adsorption capacity of the composites was measured using thermogravimetry and the SO2 adsorption characteristics were also investigated. In the low temperature region (50–200 °C), the MnO2/AC composite exhibits good SO2 trap performance and the MnO2 conversion of the composite is significantly improved. It was found that the SO2 adsorption on the MnO2/AC composite is a chemisorption process. The experimental data for SO2 adsorption on the MnO2/AC composite could fit the Freundlich model well. Changes in the thermodynamic parameters were determined. The calculated values of ΔG0 and ΔH0 indicate that the SO2 adsorption on the MnO2/AC composite is spontaneous and thermodynamically favorable.
Introduction
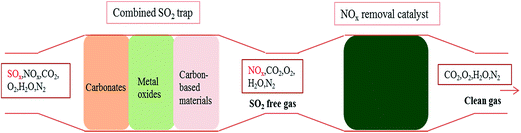
The removal of sulfur dioxide (SO2) from combustion exhaust (especially from diesel power plants and marine ships) is of particular interest for two main reasons: SO2 has detrimental effects on human health and the environment, and SO2 can deactivate NOx removal catalysts.1–4 To solve these issues caused by large amounts of SO2 emissions from diesel engine exhausts, significant efforts have been contributed to removing SO2 since the compact SO2 trap device upstream of NOx conversion device was proposed to improve the longevity of NOx removal catalysts by working against SO2 poisoning.5–7 The temperature of diesel engine exhaust is over a wide range from 200 °C to 650 °C when the engine is working. So, most desulfurization materials are focused on the desulfurization performance at 200–650 °C for a compact SO2 trap, such as calcined limestone,8 MgO9 and hydrotalcite-like compounds.10At the beginning of ignition, as the diesel is incompletely combusted, the diesel engine exhaust is in a low temperature region from 30 °C to 200 °C. Therefore, the temperature of the diesel engine exhaust is under a very wide range from 30 °C to 650 °C. Based on fundamental studies, it has been found that carbonates have good reactivity with SO2 at 650 °C, but the desulfurization rate declines below 400 °C for the reason that the reaction activity is limited by decarbonation.3 Metal oxides with the sulfate reaction path (MxOy + ySO2 + 0.5yO2 → Mx(SO4)y) exhibit good SO2 capture performance over the temperature range of 200 °C to 450 °C.11 Carbon-based materials have good desulfurization performance at 100 °C.12 However, the investigated desulfurization materials do not have a high enough SO2 capture capacity for their application in a compact SO2 trap under the wide temperature range. Therefore, a combined SO2 trap is proposed to completely capture SO2 in the temperature range of 30 °C to 650 °C. The combined SO2 trap has three parts: high-temperature materials (carbonates), middle-temperature materials (metal oxides) and low-temperature materials (carbon-based materials), as shown in the Fig. 1.
To enhance the performance of the combined SO2 trap, the improvement of low temperature sulfate activity of desulfurization materials for compact SO2 traps is needed. However, limited studies on desulfurization materials with low temperature activation have been reported. Nishioka7 aimed to improve the reaction activity of SO2 trap catalysts with a noble metal under low temperature conditions. Kylhammar13 investigated the SO2 trapping capacity of CeO2-based materials at 250 °C and the fresh sample could store about 19 mgSO2 gCeO2−1. Rubio12 studied the SO2 removal performance of coal fly ash based carbons under flue gas conditions and the amount of SO2 removed was 13 mg g−1 for the activated sample at 100 °C. Tseng14 investigated the SO2 reaction activity of copper oxide supported on activated carbon in the low temperature region. Activated carbon has various outstanding advantages, such as high adsorption capacity, high stability, low cost, low density and easy accessibility, and activated carbon-based materials have promising prospects for their use as desulfurization materials under low temperature conditions. Furthermore, it is reported that highly dispersed metal oxide particles (especially particles in the nanoscale) on activated carbon can significantly improve the catalyst activity at low temperature.15
In our previous studies,1,16 manganese dioxide (MnO2) was focused on as a candidate for the material of a compact SO2 trap in the low temperature region. However, in low temperature conditions, the sulfate ratios of the material are very small because of the aggregation of the MnO2 materials. Furthermore, in the present work, manganese dioxide/activated carbon composites were prepared by translating potassium permanganate into MnO2 which formed composites with residual carbon material using the redox deposition method and carbon as a reducer. The basic SO2 capture capacity of the composites was measured using a thermogravimetry (TG) device in the low temperature region (50–200 °C) with a 2 L min−1 gas flow containing 500 ppm SO2 in nitrogen, and the adsorption characteristics of the MnO2/AC composites toward SO2 at low temperature were also investigated.
Experimental section
Materials
The chemicals, activated carbon (BET surface area, 598 m2 g−1) and potassium permanganate, were purchased from Beijing Chemical Co., Ltd., People’s Republic of China and were of analytical reagent grade.In this experiment, MnO2/AC composites were synthesized using a simple redox deposition method.17 A 250 mL three-necked round-bottomed flask was placed in a constant temperature oil bath as the reactor. 0.5 g of activated carbon was blended with 50 mL of 0.1 mol L−1 potassium permanganate (KMnO4) solution in the flask. The flask was kept at a constant temperature in the oil bath. Then the suspension containing activated carbon was refluxed at 140 °C with sustained magnetic stirring. After 10 h, the mixture was filtered, washed with distilled water several times to remove the residual KMnO4, dried at 120 °C for 8 h, and then kept in the desiccator until use.
Characterization
In this study, the specific surface area, pore volume distribution, and surface structure were analyzed to assess the physical characteristics of the target materials. The specific surface area of these samples was measured using Brunauer–Emmett–Teller (BET) analysis with the nitrogen adsorption uptake at the boiling point of nitrogen which is 77 K using a capacitive measurement method. The pore diameter was measured using nitrogen physisorption under a normal relative pressure of 0.1–1.0 using the Barrett–Joyner–Halenda (BJH) method. Surface observation of the samples was conducted by scanning electron microscopy (SEM, Hitachi S-4800). The metal oxide yield of the composite was carried out utilizing a wavelength dispersive sequential X-ray fluorescence spectrometry (XRF, AxiosmAX Petro). Fourier transform infrared (FTIR) spectra were recorded using a Tensor 27 spectrometer with the KBr pellet method. The crystal structures were further determined using X-ray diffraction (XRD, X’Pert Pro MPD, Cu Kα radiation). X-ray photoelectron spectroscopy (XPS) was conducted to determine the chemical composition and functional groups using an XSAM-800 spectrometer (Kratos, UK) with Al (1486.6 eV) under ultrahigh vacuum (UHV) at 12 kV and 15 mA. Energy calibration was performed by recording the core level spectra of Au 4f7/2 (84.0 eV) and Ag 3d5/2 (368.30 eV).SEM images of AC and the MnO2/AC composite are shown in Fig. 2. It can be seen that a large number of MnO2 nanoparticles are uniformly formed and highly dispersed on the AC surface (Fig. 2b). Fig. 2c shows a magnified SEM image of the MnO2/AC composite. The diameters of the uniform MnO2 nanoparticles are around 100 nm. The FTIR spectrum of AC is illustrated in Fig. 3a; the peaks around 1230 cm−1 correspond to the C–H stretch vibration,18 bands at about 1570 cm−1 denote O–H bending vibrations,19 the peaks around 2360 cm−1 correspond to the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C stretching vibration,20 and the bands around 3400 cm−1 should be attributed to the O–H stretching vibration.21 From the FTIR spectra of the MnO2/AC composite shown in Fig. 3a and b the relatively sharp peak around 525 cm−1 should be ascribed to the Mn–O and Mn–O–Mn vibrations in octahedral MnO2 (ref. 22). XRD analysis (Fig. 4) confirmed the MnO2 nanoparticles. The diffraction peaks of as-synthesized MnO2/AC were similar to those of ramsdellite phase MnO2 (JCPDS 42-1316), where the reflection peak of layered AC had almost disappeared. This result indicated that homogeneous composites were formed on the AC surface, and were covered by MnO2 nanoparticles. The surface area (calculated using Brunauer–Emmett–Teller (BET) theory) of the MnO2/AC composite is 278 m2 g−1. The average pore diameter of the MnO2/AC composite is 1.2 nm, and its pore volume (calculated using Barrett–Joyner–Halenda (BJH) theory and Horvath–Kawazoe (HK) theory) is 0.34 cm3 g−1. TG/DSC profiles of parent AC and the MnO2/AC composite in the air are shown in Fig. 5. It can be seen that, a sharp weightlessness of the parent AC around 500 °C can be clearly observed, which is mainly caused by the combustion of carbon. As the temperature of carbon combustion of the MnO2/AC composite decreased to around 300 °C, the MnO2/AC composite shows high oxidation capacity.
C stretching vibration,20 and the bands around 3400 cm−1 should be attributed to the O–H stretching vibration.21 From the FTIR spectra of the MnO2/AC composite shown in Fig. 3a and b the relatively sharp peak around 525 cm−1 should be ascribed to the Mn–O and Mn–O–Mn vibrations in octahedral MnO2 (ref. 22). XRD analysis (Fig. 4) confirmed the MnO2 nanoparticles. The diffraction peaks of as-synthesized MnO2/AC were similar to those of ramsdellite phase MnO2 (JCPDS 42-1316), where the reflection peak of layered AC had almost disappeared. This result indicated that homogeneous composites were formed on the AC surface, and were covered by MnO2 nanoparticles. The surface area (calculated using Brunauer–Emmett–Teller (BET) theory) of the MnO2/AC composite is 278 m2 g−1. The average pore diameter of the MnO2/AC composite is 1.2 nm, and its pore volume (calculated using Barrett–Joyner–Halenda (BJH) theory and Horvath–Kawazoe (HK) theory) is 0.34 cm3 g−1. TG/DSC profiles of parent AC and the MnO2/AC composite in the air are shown in Fig. 5. It can be seen that, a sharp weightlessness of the parent AC around 500 °C can be clearly observed, which is mainly caused by the combustion of carbon. As the temperature of carbon combustion of the MnO2/AC composite decreased to around 300 °C, the MnO2/AC composite shows high oxidation capacity.
 | ||
| Fig. 2 (a) SEM image of parent AC, (b) SEM image of the MnO2/AC composite, and (c) enlarged image of MnO2 nanoparticles from panel (b). | ||
Thermogravimetric measurements
Thermogravimetry (TG) was used in this study to measure the effectiveness of the sulfate reaction of the MnO2/AC composite. Fig. 6 shows a schematic drawing of the TG analysis experiment. An amount of 50 mg of a sample on a quartz crucible was slowly (10 K min−1) heated to the target temperature under an atmosphere of nitrogen, and maintained under this condition for about 2 hours. Reactant gas flow (SO2 in base N2) was controlled using a mass flow controller. The total flow gas rate was 2 L min−1. During SO2 adsorption, the gas stream was passed over the sample at the target temperature for 2 hours. The reaction temperature of the TG experiment ranged from 50 °C to 250 °C.The SO2 capture performance of the samples was measured. The SO2 capture performance per unit mass P and the conversion of MnO2 X(t) are expressed by the following equations:
 | (1) |
 | (2) |
Results and discussion
Basic sulfate performance of the MnO2/AC composite
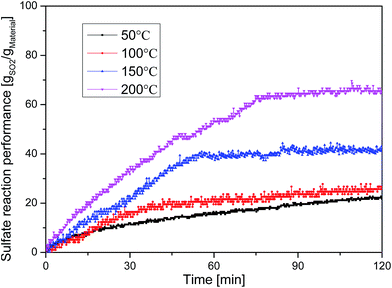
Fig. 7 shows the influence of the sulfate reaction temperature on the SO2 capture performance of the MnO2/AC composite. The SO2 capture performance of the MnO2/AC composite was investigated from 50 °C to 200 °C. From the results shown in Fig. 7, the SO2 capture performance of the MnO2/AC composite increased with the experimental temperature. The SO2 capture capacities (mg g−1) of the MnO2/AC composite and pure AC at different temperature conditions are listed in Table 1. At 200 °C, the composite exhibited good SO2 capture performance with an absorbance of about 65.6 gSO2 gComposite−1 and the SO2 capture performance of AC was 2.1 gSO2 gAC−1. As pure AC exhibited a higher SO2 capture performance at a lower temperature and captured 15.4 gSO2 gAC−1 at 50 °C, the MnO2/AC composite had a very high SO2 capture performance (21.7 gSO2 gComposite−1) at 50 °C. In comparison with investigated low temperature desulfurization materials, such as CuO/AC (below 10 mg g−1)14 and coal fly ash (13 mg g−1),12 the MnO2/AC composite exhibited a high SO2 capture performance over the low temperature range from 50 °C to 200 °C. It was found that manganese oxide impregnated on activated carbon could play a key role in SO2 removal.23 Based on the results shown in Fig. 2, it is evident that the oxidative activity of the MnO2/AC composite was significantly improved because the MnO2 nanoparticles are highly dispersed on activated carbon by the redox deposition method.24| Sample | 50 °C | 100 °C | 150 °C | 200 °C |
|---|---|---|---|---|
| MnO2/AC composite | 21.7 | 25.7 | 43.1 | 65.6 |
| AC | 15.4 | 8.6 | 3.3 | 2.1 |
The surface functional groups of activated carbon are very important factors for SO2 removal.23 The XPS analyses were carried out to determine the functional groups on pure AC and the MnO2/AC composite, as shown in Fig. 8. The C 1s pattern of all samples included graphitizing carbon (C–C/C–H), phenolic (C–O), carbonyl carbon (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and transition (π–π*) with binding energies at around 285, 286, 288 and 290 eV, respectively.23 The content of π–π* transition in the MnO2/AC composite slightly increased compared to that in AC. It was reported that the functional group of π–π* transition with basic nature was more favorable for SO2 removal.25 Thus, the change of surface functional group was responsible for the better SO2-capture performance of the MnO2/AC composite.
O) and transition (π–π*) with binding energies at around 285, 286, 288 and 290 eV, respectively.23 The content of π–π* transition in the MnO2/AC composite slightly increased compared to that in AC. It was reported that the functional group of π–π* transition with basic nature was more favorable for SO2 removal.25 Thus, the change of surface functional group was responsible for the better SO2-capture performance of the MnO2/AC composite.
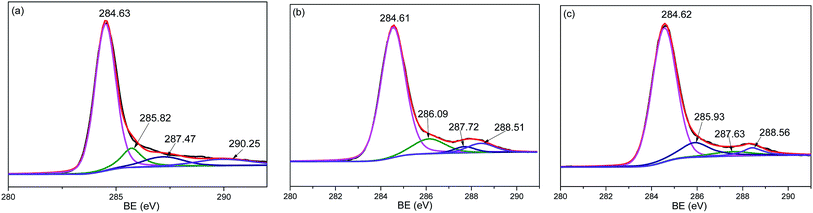 | ||
| Fig. 8 C 1s patterns of XPS spectra: (a) prepared AC, (b) MnO2/AC composite before desulfurization, and (c) MnO2/AC composite after desulfurization. | ||
In order to further study the chemical composition information of the MnO2/AC composite after SO2 removal, XPS analysis was carried out to clarify the Mn species on activated carbon; the Mn 2p XPS patterns of the MnO2/AC composite are shown in Fig. 9. Before desulfurization, the Mn 2p3/2 region of the MnO2/AC composite consisted of one sharp and strong peak at 641.90 eV (Fig. 9a), attributed to Mn4+ (641.1–642.4 eV) in MnO2,26 indicating that MnO2 was formed on the surface of activated carbon. After desulfurization, the peak at 642.60 eV (Fig. 9b) is characteristic of Mn2+.27 Fig. 9c shows S 2p XPS spectra of the MnO2/AC composite after desulfurization. The S 2p3/2 binding energy of the MnO2/AC composite was 168.67 eV and could be assigned to SO42−, indicating that MnO2 on the surface of the activated carbon was transformed to MnSO4 during the desulfurization process.28
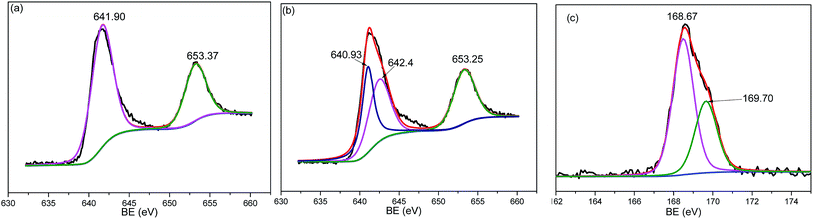 | ||
| Fig. 9 Mn 2p patterns of MnO2/AC: (a) before desulfurization, and (b) after desulfurization. (c) S 2p pattern of MnO2/AC after desulfurization. | ||
Fig. 10 shows a comparison of the sulfate ratio of the MnO2/AC composite excluding the SO2 capture amount of the pure AC with that of MnO2 at 50 °C, 100 °C, 150 °C and 200 °C. The sulfate ratio of the composite was significantly improved at low temperature conditions compared with that of pure MnO2. After desulfurization at the low temperatures of 50 °C and 100 °C, the sulfate ratio of the MnO2/AC composite was 80% and 65% higher than that of MnO2, respectively. It was indicated that the deposition of MnO2 on the surface of activated carbon by a simple redox deposition method was effective in enhancing SO2 removal at low temperatures for a combined SO2 trap for a diesel exhaust system.
 | ||
| Fig. 10 Comparison of the sulfate ratio of the MnO2/AC composite with that of MnO2 at low temperatures from 50 °C to 200 °C. | ||
Effect of the precursor concentration on SO2 adsorption capacity
The concentration of KMnO4 was varied from 0.01 mol L−1 to 0.5 mol L−1. Table 2 displays the textural properties of MnO2/AC composites. After the redox deposition process, the pore volume of the product MnO2/AC composite was much smaller than the pure AC (0.79 cm3 g−1) and the BET surface area as well as the pore volume of the MnO2/AC composites decreased along with the rise in the concentration of KMnO4 because the uniform MnO2 nanoparticles plugged the pores of the AC surface, as is shown in Table 2.| Sample | KMnO4 concentration (mol L−1) | BET surface area (m2 g−1) | Pore volume (cm3 g−1) |
|---|---|---|---|
| AC0 | 0 | 598 | 0.79 |
| AC1 | 0.01 | 473 | 0.64 |
| AC2 | 0.05 | 345 | 0.41 |
| AC3 | 0.1 | 278 | 0.34 |
| AC4 | 0.2 | 186 | 0.15 |
| AC5 | 0.3 | 91 | 0.09 |
| AC6 | 0.5 | 45 | 0.03 |
The MnO2 loading amount of the prepared composites was calculated utilizing Wavelength Dispersive Sequential X-ray Fluorescence Spectrometry (XRF, AxiosmAX Petro). In the study, the loading amount of MnO2 ranged from 5% to 40%. In Fig. 11, the loading amount of MnO2 increased along with the rise of the concentration of KMnO4 as expected. From the results in Fig. 11, it is found that at high concentrations the increasing rate was much lower than at low concentrations, the reason of which could be that, at lower concentrations, the degree of supersaturation was not adequate to generate abundant growth units on the AC surface. At higher concentrations the degree of supersaturation was adequate, so the loading amount of MnO2 grew much slower with the increase of KMnO4 concentration.15 It can be concluded that, the supersaturation was intensified with the increase of the KMnO4 concentration, leading to quick nucleation and a high growth rate of the loading amount of MnO2 and a high density of MnO2 nanoparticles was dispersed on the AC surface.
 | ||
| Fig. 11 Effect of the KMnO4 concentration on SO2 adsorption capacity of the as-prepared MnO2/AC composites at 100 °C, for 500 ppm SO2 in nitrogen. | ||
Fig. 11 also displays the SO2 capture capacity of the as-prepared MnO2/AC composites with a variation of precursor concentrations at 100 °C, for 500 ppm SO2 in nitrogen. The AC composites reveal a tremendously improved adsorption capacity in comparison to the parent AC. The SO2 capture capacity of the composites initially increased with the rising KMnO4 concentration and reached a maximum value when the KMnO4 concentration was 0.2 mol L−1. The maximum SO2 capture capacity of the as-prepared MnO2/AC composites was 29 mgSO2 gMaterial−1, which was significantly higher than for other low temperature desulfurization materials (such as vanadium/activated carbon reported by Carabineiro,29 coal fly ash reported by Rubio12 and CuO/AC reported by Tseng14). However, when the KMnO4 concentration further increased, the desulfurization capacity decreased instead. A similar tendency was also observed by Carabineiro29 and Sumathi.30 The SO2 capture capacity presented an obvious decrease at the high KMnO4 concentration, mostly because the accumulation of MnO2 nanoparticles greatly reduced the surface area, pore volume and the amount of effective active sites.15
Equilibrium adsorption isotherm
For heterogeneous adsorption on the surface of a material, the three most conventional sorption isotherm models used to represent the collected equilibrium data are the Langmuir, Freundlich and BET models. This study employed these three models to describe the equilibrium adsorption. The Langmuir model assumes that equilibrium is obtained when a monolayer of the adsorbate-molecules (SO2) saturates the adsorbent (MnO2/AC composite). The expression of the Langmuir model can be written as:
 | (3) |
 | (4) |
The values of qm and b can be determined from the slope and intercept of a linear plot of Ce/q versus Ce.
The Freundlich model is an empirical equation that assumes heterogeneous adsorption owing to the diversity of adsorption sites. The Freundlich model can be described by the following equation:
| q = KfCe(1/n) | (5) |
The linear form of the Freundlich model takes the form:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) q = ln q = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf + (1/n)ln Kf + (1/n)ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (6) |
where the values of Kf and n can be obtained from the intercept and the slope of a linear plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) q versus ln
q versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce, respectively.
Ce, respectively.
The Brunauer–Emmett–Teller (BET) model was used to fit data that follow multilayer adsorption. The expression of the BET model can be written as:
 | (7) |
Among these three conventional sorption isotherm models, the Freundlich model is very applicable when the adsorbate is strongly adsorbed on the surface of the adsorbent where a logarithmic fall in the enthalpy of adsorption with surface coverage has occurred. To determine the shape of the adsorption equilibrium for this system, the type of interaction between SO2 gas and the MnO2/AC composite should be understood.31 To obtain further insight into the SO2 adsorption by the MnO2/AC composite, Fourier transform infrared (FTIR) spectra were conducted in this study. The FTIR spectrum of the MnO2/AC composite after desulfurization is illustrated in Fig. 12b, in which it can be seen that the intensity of the band at 1102 cm−1 is due to the stretching motion of adsorbed sulfate on the surface of desulfurization materials.32 It can be concluded that SO2 adsorption by the MnO2/AC composite is a chemisorption process and the sulfate is formed. Based on the results of Fig. 2b and 12, as the uniformly MnO2 nanoparticles are highly dispersed on the AC surface, the SO2 gas can easily cover the surface of the MnO2/AC composite and it can be strongly chemisorbed by the absorbent to form sulfate. Therefore, the Freundlich model could be applicable for the SO2 adsorption by the MnO2/AC composite.
 | ||
| Fig. 12 FTIR spectra of (a) the MnO2/AC composite and (b) the MnO2/AC composite after desulfurization. | ||
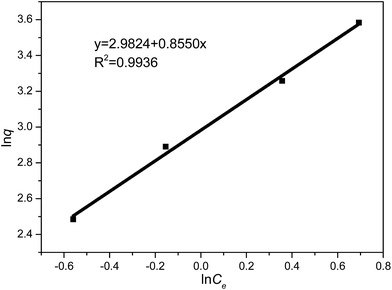
In order to determine the constants of the Freundlich model for the SO2 adsorption by the MnO2/AC composite, the equilibrium adsorption capacity was measured under different SO2 gas concentration conditions (200 ppm, 300 ppm, 500 ppm and 700 ppm) at 373 K. The corresponding Freundlich plot of the SO2 adsorption capacity of the MnO2/AC composite is shown in Fig. 13. From the results of Fig. 13, it can be seen that the Freundlich model fits the experimental data reasonably well, and the value of R-square is as high as 0.9936. A Freundlich constant, n related to the sorption intensity, of 1.170 was calculated from the intercept and the slope of the Freundlich model. Compared with the value (1.059) of the Freundlich constant n for zeolitic tuff reported by Al-Harahsheh,31 it can be concluded that the MnO2/AC composite exhibits high activity for SO2 adsorption.
Adsorption thermodynamics
The thermodynamic parameters could provide further information of inherent energetic changes of the SO2 adsorption by the MnO2/AC composite. To determine the thermodynamic parameters such as heat of adsorption (ΔH0), entropy (ΔS0) changes and the free energy of the process (ΔG0), the following equations were used:
ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf Kf
| (8) |
 | (9) |
| Temperature (K) | ΔG0 (kJ mol−1) | ΔH0 (kJ mol−1) | ΔS0 [J mol−1 K−1] |
|---|---|---|---|
| 323 | −8.01 | ||
| 373 | −9.25 | ||
| 423 | −12.23 | 14.30 | 62.97 |
| 473 | −15.43 | ||
| 523 | −18.73 |
Conclusions
In this study, MnO2/AC composites have been successfully synthesized using the redox deposition method. The uniform MnO2 nanoparticles are highly dispersed on the AC surface. The MnO2/AC composite exhibits good SO2 trap performance and the MnO2 conversion is improved over a low temperature range from 50 °C to 200 °C. The deposition of MnO2 on the surface of activated carbon was effective in enhancing SO2 removal at low temperature conditions for a combined SO2 trap for a diesel exhaust system. The SO2 capture capacity of the MnO2/AC composite initially increased and then decreased with the precursor concentration rising. Furthermore, the type of interaction between SO2 gas and the MnO2/AC composite was determined, and the results indicated that the SO2 adsorption on the MnO2/AC composite is a chemisorption process. The Freundlich model fit the experimental data very well. The calculated value of the Freundlich constant (n) is 1.170, indicating high activity for SO2 adsorption on the MnO2/AC composite. The calculated values of ΔH0 and ΔS0 are 14.30 kJ mol−1 and 62.97 J mol−1 K−1, respectively. Positive ΔH0 and ΔS0 values suggest that the SO2 adsorption on the MnO2/AC composite is endothermic and the ΔG0 values are negative, indicating that the SO2 adsorption on the MnO2/AC composite is spontaneous and favorable.Acknowledgements
This research was supported by National Natural Science Foundation of China (NSFC) through International (Regional) Cooperation and Exchange Projects (21550110494).References
- Y. Osaka, T. Kito, N. Kobayashi, S. Kurahara, H. Huang, H. Yuan and Z. He, Removal of sulfur dioxide from diesel exhaust gases by using dry desulfurization MnO2 filter, Sep. Purif. Technol., 2015, 150, 80–85 CrossRef CAS
.
- J. Ding, Q. Zhong and S. Zhang, Simultaneous removal of NOx and SO2 with H2O2 over Fe based catalysts at low temperature, RSC Adv., 2014, 4(11), 5394–5398 RSC
.
- H. Moshiri, B. Nasernejad, H. Ale Ebrahim and M. Taheri, Solution of coupled partial differential equations of a packed bed reactor for SO2 removal by lime using the finite element method, RSC Adv., 2015, 5(23), 18116–18127 RSC
.
- Y. Zhao and G. Hu, Removal of SO2 by a mixture of caprolactam tetrabutyl ammonium bromide ionic liquid and sodium humate solution, RSC Adv., 2013, 3(7), 2234–2240 RSC
.
- L. Limousy, H. Mahzoul, J. F. Brilhac, F. Garin, G. Maire and P. Gilot, A study of the regeneration of fresh and aged SOx adsorbers under reducing conditions, Appl. Catal., B, 2003, 45(3), 169–179 CrossRef CAS
.
- L. Limousy, H. Mahzoul, J. F. Brilhac, P. Gilot, F. Garin and G. Maire, SO2 sorption on fresh and aged SOx traps, Appl. Catal., B, 2003, 42(3), 237–249 CrossRef CAS
.
- H. Nishioka, K. Yoshida, T. Asanuma and T. Fukuma, Development of clean diesel NOx after-treatment system with sulfur trap catalyst, SAE Int. J. Fuels Lubr., 2010, 3, 30–36 CrossRef CAS
.
- R. H. Borgwardt, Kinetics of the reaction of sulfur dioxide with calcined limestone, Environ. Sci. Technol., 1970, 4(1), 59–63 CrossRef
.
- F. Fang, Z. S. Li, N. S. Cai, X. Y. Tang and H. T. Yang, AFM investigation of solid product layers of MgSO4 generated on MgO surfaces for the reaction of MgO with SO2 and O2, Chem. Eng. Sci., 2011, 66(6), 1142–1149 CrossRef CAS
.
- M. Cantu, E. Lopez-Salinas and J. S. Valente, SOx removal by calcined MgAIFe hydrotalcite-like materials: Effect of the chemical composition and the cerium incorporation method, Environ. Sci. Technol., 2005, 39(24), 9715–9720 CrossRef CAS PubMed
.
- X. Liu, Y. Osaka, H. Huang, A. Kodama, Z. He, Huhetaoli, X. Yang and Y. Chen, Development of high-performance SO2 trap materials in the low-temperature region for diesel exhaust emission control, Sep. Purif. Technol., 2016, 162, 127–133 CrossRef CAS
.
- B. Rubio and M. T. Izquierdo, Coal fly ash based carbons for SO2 removal from flue gases, Waste Manage., 2010, 30(7), 1341–1347 CrossRef CAS PubMed
.
- L. Kylhammar, P.-A. Carlsson, H. H. Ingelsten, H. Grönbeck and M. Skoglundh, Regenerable ceria-based SOx traps for sulfur removal in lean exhausts, Appl. Catal., B, 2008, 84(1–2), 268–276 CrossRef CAS
.
- H. H. Tseng and M. Y. Wey, Study of SO2 adsorption and thermal regeneration over activated carbon-supported copper oxide catalysts, Carbon, 2004, 42(11), 2269–2278 CrossRef CAS
.
- Z. Yan, J. P. Wang, R. Q. Zou, L. L. Liu, Z. T. Zhang and X. D. Wang, Hydrothermal synthesis of CeO2 nanoparticles on activated carbon with enhanced desulfurization activity, Energy Fuels, 2012, 26(9), 5879–5886 CrossRef CAS
.
- Y. Osaka, K. Yamada, T. Tsujiguchi, A. Kodama, H. Huang and Z. He, Study on the optimized design of DeSOx filter operating at low temperature in diesel exhaust, J. Chem. Eng. Jpn., 2014, 47, 555–560 CrossRef CAS
.
- Z. J. Fan, J. Yan, T. Wei, L. J. Zhi, G. Q. Ning, T. Y. Li and F. Wei, Asymmetric
supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density, Adv. Funct. Mater., 2011, 21, 2366–2375 CrossRef CAS
.
- A.-N. A. El-Hendawy, Influence of HNO3 oxidation on the structure and adsorptive properties of corncob-based activated carbon, Carbon, 2003, 41(4), 713–722 CrossRef CAS
.
- H. Y. Chu, Q. Y. Lai, L. Wang, J. F. Lu and Y. Zhao, Preparation of MnO2/WMNT composite and MnO2/AB composite by redox deposition method and its comparative study as supercapacitive materials, Ionics, 2010, 16(3), 233–238 CrossRef CAS
.
- L. Wang, J. Zhang, R. Zhao, Y. Li, C. Li and C. Zhang, Adsorption of Pb(II) on activated carbon prepared from Polygonum orientale Linn.: Kinetics, isotherms, pH, and ionic strength studies, Bioresour. Technol., 2010, 101(15), 5808–5814 CrossRef CAS PubMed
.
- C. Aguilar, R. García, G. Soto-Garrido and R. Arriagada, Catalytic wet air oxidation of aqueous ammonia with activated carbon, Appl. Catal., B, 2003, 46(2), 229–237 CrossRef CAS
.
- A. Yuan and Q. Zhang, A novel hybrid manganese dioxide/activated carbon supercapacitor using lithium hydroxide electrolyte, Electrochem. Commun., 2006, 8(7), 1173–1178 CrossRef CAS
.
- Y.-F. Qu, J.-X. Guo, Y.-H. Chu, M.-C. Sun and H.-Q. Yin, The influence of Mn species on the SO2 removal of Mn-based activated carbon catalysts, Appl. Surf. Sci., 2013, 282, 425–431 CrossRef CAS
.
- Q. Tang, X. Huang, Y. Chen, T. Liu and Y. Yang, Characterization and catalytic application of highly dispersed manganese oxides supported on activated carbon, J. Mol. Catal. A: Chem., 2009, 301(1–2), 24–30 CrossRef CAS
.
- L. Yang, X. Jiang, Z.-S. Yang and W.-J. Jiang, Effect of MnSO4 on the Removal of SO2 by Manganese-Modified Activated Coke, Ind. Eng. Chem. Res., 2015, 54(5), 1689–1696 CrossRef CAS
.
- Y. Wu, Y. Lu, C. Song, Z. Ma, S. Xing and Y. Gao, A novel redox-precipitation method for the preparation of α-MnO2 with a high surface Mn4+ concentration and its activity toward complete catalytic oxidation of o-xylene, Catal. Today, 2013, 201, 32–39 CrossRef CAS
.
- Y. Xia, L. Meng, Y. Jiang, Y. Zhang, X. Dai and M. Zhao, Facile preparation of MnO2 functionalized baker’s yeast composites and their adsorption mechanism for Cadmium, Chem. Eng. J., 2015, 259, 927–935 CrossRef CAS
.
- M. Y. Smirnov, A. V. Kalinkin, A. V. Pashis, A. M. Sorokin, A. S. Noskov, V. I. Bukhtiyarov, K. C. Kharas and M. A. Rodkin, Comparative XPS study of Al2O3 and CeO2 sulfation in reactions with SO2, SO2 + O2, SO2 + H2O, and SO2 + O2 + H2O, Kinet. Catal., 2003, 44(4), 575–583 CrossRef CAS
.
- S. A. C. Carabineiro, A. M. Ramos, J. Vital, J. M. Loureiro, J. J. M. Órfão and I. M. Fonseca, Adsorption of SO2 using vanadium and vanadium-copper supported on activated carbon, Catal. Today, 2003, 78(1–4), 203–210 CrossRef CAS
.
- S. Sumathi, S. Bhatia, K. T. Lee and A. R. Mohamed, Selection of best impregnated palm shell activated carbon (PSAC) for simultaneous removal of SO2 and NOx, J. Hazard. Mater., 2010, 176(1–3), 1093–1096 CrossRef CAS PubMed
.
- M. Al-Harahsheh, R. Shawabkeh, M. Batiha, A. Al-Harahsheh and K. Al-Zboon, Sulfur dioxide removal using natural zeolitic tuff, Fuel Process. Technol., 2014, 126(0), 249–258 CrossRef CAS
.
- L. Zhao, X. Li, X. Quan and G. Chen, Effects of surfacefeatures on sulfur dioxide adsorption on calcined NiAl hydrotalcite-like compounds, Environ. Sci. Technol., 2011, 45(12), 5373–5379 CrossRef CAS PubMed
.
- V. K. Gupta, I. Ali, Suhas and D. Mohan, Equilibrium uptake and sorption dynamics for the removal of a basic dye (basic red) using low-cost adsorbents, J. Colloid Interface Sci., 2003, 265(2), 257–264 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2016 |