Silver-catalyzed synthesis of 2-arylvinylphosphonates by cross-coupling of β-nitrostyrenes with H-phosphites†
Abstract
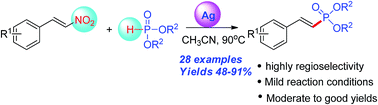
An efficient protocol for stereoselective synthesis of 2-arylvinylphosphonates has been developed via AgNO3-catalyzed cross-coupling of β-nitrostyrenes with dialkyl H-phosphites under mild conditions. By losing the nitro group of β-nitrostyrenes, the reaction proceeds smoothly and could provide the desired products with moderate to good yield.

 Please wait while we load your content...
Please wait while we load your content...