Catalytic performance of layered double hydroxide nanosheets toward phenol hydroxylation†
Abstract
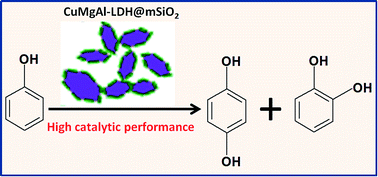
Layered double hydroxides (LDHs) materials have shown promising behaviour in heterogeneous catalysis, and increasing the amount of exposed active sites is crucial to enhance their catalytic performance. In this work, CuMgAl-LDH@mSiO2 nanosheets were prepared by delaminating the CuMgAl(NO3)-LDH microcrystals followed by coating a layer of SiO2. Structural characterizations based on SEM and BET display their slice morphology with average thickness of 5.2 nm and a large specific surface area of 244.3 m2 g−1. This gives rise to excellent catalytic performance toward phenol hydroxylation (conversion: 45.6%; selectivity: 97.3%). Especially, the normalized activity value is as high as 330.8 (mol phenol/mol Cu2+) with the efficiency of peroxide up to 91.1%. This is, to the best of our knowledge, the highest value for Cu-based heterogeneous catalysts in this reaction. This work demonstrates the unique structural feature of CuMgAl-LDH@mSiO2 nanosheets with efficient exposure of active sites and its remarkably enhanced catalytic activity toward phenol hydroxylation.

 Please wait while we load your content...
Please wait while we load your content...