The effect of pH on the properties of 3D welan gum–graphene oxide composite hydrogels and their excellent adsorption capacity†
Jianmei Jiaoa,
Xia Xin*ab,
Jinglin Shenb,
Zhaohua Songa,
Zengchun Xiea and
Guiying Xuab
aNational Engineering Technology Research Center for Colloidal Materials, Shandong University, Shanda nanlu No. 27, Jinan, 250100, P. R. China. E-mail: xinx@sdu.edu.cn; Fax: +86-531-88361008; Tel: +86-531-88363597
bKey Laboratory of Colloid and Interface Chemistry (Shandong University), Ministry of Education, Shanda nanlu No. 27, Jinan, 250100, P. R. China
First published on 29th September 2016
Abstract
In this article, welan gum–graphene oxide (GO) composite hydrogels were prepared by simple self-assembly of these two components in aqueous media and the effects of pH on their properties were systematically studied. The welan gum–GO hybrid hydrogels with different pH have been thoroughly characterized using TEM, FE-SEM, FT-IR spectroscopy, XRD and rheological measurements. It can be observed that the microstructures of welan–gum hydrogels can be tailored by varying the pH value and swelling properties analysis shows that the swelling ratio increases with pH. Moreover, the welan gum–GO hybrid hydrogels exhibited improved adsorption properties for water-soluble dyes under acidic conditions than under basic conditions. At the same time, adsorption isotherms and kinetics were also studied. Thus, it is expected that the GO-based composite hydrogels can act as potential adsorbents and have promising applications in the field of waste water treatment by adjusting pH.
Introduction
Welan gum, a new microbial polysaccharide produced by Alcaligenes sp., consists of a pentasaccharide repeating unit, b-1,3-D-glucopyranosyl, b-1,4-D-glucuronopyranosyl, b-1,4-D-glucopyranosyl, x-1,4-L-rhamnopyranosyl, and a single monosaccharide side-chain at O-3 of the 4-linked glucopyranosyl.1–4 As a renewable material, welan gum is both biocompatible and biodegradable and it has potential industrial applications in food, concrete, petroleum, ink and other industries, especially in the oil industry; welan gum is expected to become a novel oil recovery agent due to its excellent rheological properties.5Because of their unique mechanical and electrical properties, carbon materials have recently attracted significant interest and have a wide range of potential applications in transistors, polymer reinforcement, and biodevices.6–8 Besides amorphous carbon, carbon nanotubes and fullerene, a new kind of carbon material-graphene oxide (GO) which has a large number of oxygen-containing groups, such as hydroxyl, epoxide and carboxyl groups on the basal planes and edges, is one of the most important derivatives of graphene. As a result, it makes GO behave like an amphiphilic macromolecule with hydrophilic edges and a more hydrophobic basal plane, and possess special hydrophilic properties which can be dispersible in water as well as other solvents.
Various researchers have focused on influencing factors that could affect the properties of graphene-based composites, among them pH is one of the most universal factors which have been involved.9,10 Currently, pH-sensitive hydrogel is one of the most widely investigated intelligent hydrogels and has attracted attention as appealing platforms for several applications such as drug release11,12 and potentiometric sensor. As one of the the most frequently applied stimuli, pH is an important environmental factor in biomedical and other systems. Lot of researches have provided that pH of GO suspension has a significant effect on its performance. For example, Qin et al. reported the sol–gel transformation of the pH-sensitive GO hydrogel and found that it was gellable in acidic media (pH < 7) via multiple non-covalent interactions, nevertheless, the GO hydrogel underwent the gel–sol transition under alkaline conditions due to the strong repulsion from the deprotonated carboxyl groups.13 Liu et al. showed the SEM micrographs of graphene/PAA hydrogels after incubation in different pH solutions. They observed different pore sizes in acidic and alkaline environment.12 Xie et al. studied the swelling ratios under various pH, then made a conclusion that the swelling ratio of GO–RCE/PVA hydrogel could increase from 150% (pH = 2) to 310% (pH = 14).14 Cheng et al. pay attention to dye adsorption of hydrogels under different pH, it was found that the adsorbed amounts for GO and GO–biopolymers increased with the increase of the pH values of the solutions and all the GO–biopolymer gels exhibited pH sensitivity and dependent adsorption capacity on these cationic molecules.15 The results reported above enlighten us that pH could affect the hydrogels that contain some specific function groups such as carboxyl, hydroxyl, amino and so on, which can bring various behaviors in different acid–base environment. Since both welan gum and GO have a certain number of carboxyl and hydroxyl, especially for GO, thus, it is speculated that welan gum/GO composites could have a sensitivity to pH and it is essential to find the optimal acid–base environment which can make the welan gum/GO composites get the best behavior by adjusting pH.
In our previous work, the welan gum–GO hybrid hydrogels were prepared by a simple self-assembly strategy and the effects of GO on the gelation of welan gum have been systematically studied.15 In this article, for better understanding the full advantage of welan gum–GO hybrid hydrogels, the effects of pH on the properties of welan gum–GO hybrid hydrogels including phase behavior, microstructure, adsorption capacity for dyes were investigated by using transmission electron microscopy (TEM), field emission scanning electron microscopy (FE-SEM), Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), UV-vis measurements, and rheological measurements. The pH-sensitive swelling behaviors, as well as the dye adsorption capacity of the welan gum–GO hybrid hydrogel were systematically studied. What we expect is to find the optimal acid–base environment for the welan gum/GO composites to achieve optimal performance.
Experimental section
Chemicals and materials
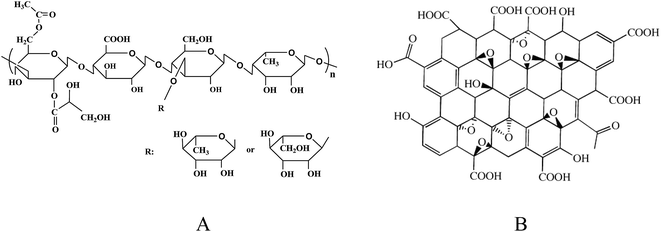
Welan gum was supplied by Hebei Hengbiao Bio-technology Co., Ltd, China. The molecular weight is about 6.6 × 105 to 9.7 × 105 g mol−1, and the intrinsic viscosity is 4479 mL g−1. Graphene oxide (diameter: 0.5–5 mm; thickness: 0.8–1.2 nm; single layer ratio: 99%; purity: 99%) was obtained from Nanjing XFNANO Materials Tech Co., Ltd and was used as received. The structures of welan gum and GO are displayed in Scheme 1. Dyes (p.a. quality) such as methylene blue (MB), methyl violet (MV), amino black 10B (AB10B), rhodamine 6G (R6G) and Chrome Azurol S (CAS) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Water used in the experiment was triply distilled by a quartz water purification system.Methods and characterizations
TEM observations were carried out on a JEOL JEM-100 CXII (Japan) at an accelerating voltage of 80 kV. For sample preparation, about 5 μL of hydrogel was placed on a ultrathin carbon-coated copper grid and the excess hydrogel was wicked away with filter paper, which was then dried by using an Near Infrared Reflection (NIR) lamp before observations. For field-emission scanning electron microscopy (FE-SEM) observations, a drop of hydrogel was placed on a silica wafer to form a thin film, which was then freeze-dried in vacuum at −55 °C. A layer of gold was sputtered on top to make the conducting surface. The wafer was observed on a JSM-6700F FE-SEM. FT-IR spectra were recorded on a VERTEX-70/70v spectrometer (Bruker Optics, Germany). The samples for FT-IR spectra were prepared by using KBr pellets mixed with the freezing-dried hydrogels. UV-vis measurements were carried out on a computer manipulated spectrometer (UV-vis 4100, Hitachi, Japan). XRD patterns of the freeze-dried samples were obtained between 5 and 90 in the 2θ scan mode (2.5 min−1) using a Rigaku D/Max 2200 PC diffractometer with Cu Kα radiation (λ = 0.15418 nm) and a graphite monochromator at room temperature.The rheological measurements were carried out on a HAAKE RS75 rheometer with a cone-plate system (Ti, diameter, 35 mm; cone angle, 1°). For the shear-dependent behavior, the viscosity measurements were carried out at shear rates ranging from 0 to 1000 s−1. For oscillatory measurements, an amplitude sweep at a fixed frequency of 1 Hz was performed prior to the following frequency sweep in order to ensure that the selected stress was in the linear viscoelastic region. The viscoelastic properties of the samples were determined using oscillatory measurements in the frequency range of 0.01–10 Hz. The samples were measured at 20.0 ± 0.1 °C with the help of a circulating water bath.
Preparation of the samples
A certain amount of welan gum was added to deionized water, swelled for one day and stirred continuously at 25 °C until dissolved. Then the samples were kept at 20 °C for two weeks before characterization. The desired amount of dried GO was exfoliated and dispersed in deionized water by sonication for 4 h.The GO dispersion and welan gum solution were mixed together at a certain ratio. Then adjust pH to 0.8, 2.3, 4.2, 6.5, 8.1, 10.6 by using 0.1 mol mL−1 HCl and 0.1 mol mL−1 NaOH, respectively. The concentration of GO and welan gum were both 0.5 mg mL−1. The mixture was shaken violently for 50 s. The composite gels were kept at 20 °C for two weeks before characterization. Finally, part of the hydrogels were freeze-dried to obtain the welan gum–GO composite gel. The structures of welan gum and GO are showed in Scheme 1.
Swelling properties analysis of the hydrogel
To measure the effect of pH on the swelling ratio of the hydrogel, the dried sample (20 mg) was immersed in various pH environments at room temperature. The swelling ratio (SR) (gram per gram) of the hydrogel was calculated using the following equation:| SR = (Wt − W0)/W0 × 100% | (1) |
Dye adsorption
To study the effect of pH value on the adsorption of dye molecules, a variety of water soluble dyes such as MB, MV, AB10B, R6G and CAS were used to study the dye adsorption capacity of the welan gum–GO composite hydrogels under different pH. In further research, R6G was chosen as a sample to study adsorption isotherm and adsorption kinetics. The adsorption capacity, in other word, the amount of dye adsorbed per unit mass of the hydrogel was evaluated by using the mass balance equation:| q = (C0 − Ce)V/m | (2) |
Results and discussion
Phase behavior of welan gum–GO hydrogels with different pH
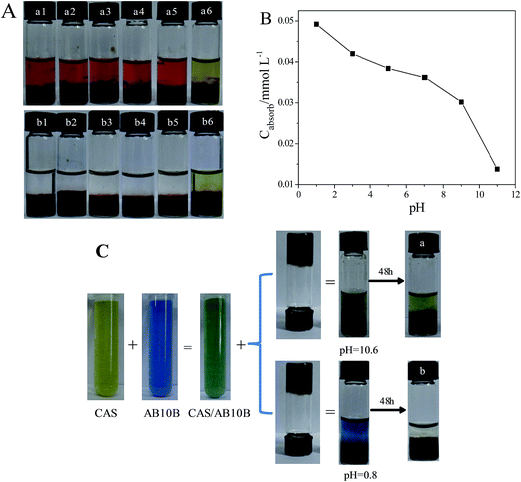
In order to study the influence of pH on the welan gum–GO hydrogels, firstly, a simple study on the phase behavior of 5 mg mL−1 welan gum and 5 mg mL−1 GO hydrogels with different pH was performed and the result was shown in Fig. 1. It can be seen that there is no obvious phase change when pH varies from 0.8 to 10.6, and all the samples remain hydrogel state in the inversion test tubes.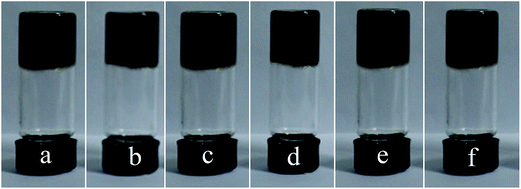 | ||
| Fig. 1 Phase behavior of 5 mg mL−1 welan gum and 5 mg mL−1 GO hydrogels with different pH (from (a–f), pH is 0.8, 2.3, 4.2, 6.5, 8.1, 10.6, respectively). | ||
Microstructures of welan gum–GO hydrogels with different pH
TEM and FE-SEM images were used to characterize the structures of the welan gum–GO composite hydrogels with different pH. From the TEM results shown in Fig. 2A–C, it can be seen that the hydrogels are stable within a wide range of pH without obvious aggregation. In order to further confirm the microstructures of the hydrogels, FE-SEM images have been taken. From the images of hydrogels at different pH, we can see that the morphologies of the welan gum–GO composite hydrogels become larger and tend to form GO structure when it is in an acid condition (Fig. 2D). However, with the increase of pH, the welan gum chains also increase. When pH reaches 10.6, the welan gum chains dominate and fold on the surface of GO can be also found (Fig. 2F). Comparing with the hydrogel at original pH (pH = 6, Fig. 2E), the network structures of welan gum–GO hydrogel become looser in alkaline condition. This is because that in alkaline condition, the negative charge on the GO surface increases, which makes the electrostatic repulsion between GO layers larger. | ||
| Fig. 2 TEM (A–C) and SEM (D–F) images of the hydrogels with 5 mg mL−1 welan gum and 5 mg mL−1 GO at pH of 0.8 (A and D), 6 (B and E) and 10.6 (C and F). | ||
FT-IR and XRD analysis
FT-IR is an important method to characterize the interactions of self-assembling supramolecular hydrogels and it can be used to investigate the interaction between welan gum and GO. As shown in Fig. 3A and B, the characteristic vibrations of GO are dominated by the intense peak of O–H groups centered at 3446 cm−1, strong C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak at 1633 cm−1, –OH bending vibration of carboxyl group at 1386 cm−1, the C–OH stretching peak at 1266 cm−1 and the C–O stretching peak at 1044 cm−1. In the spectrum of welan gum, the broad band at 3419 cm−1 corresponds to the hydroxyl groups, the peak at 1727 cm−1 is C
O peak at 1633 cm−1, –OH bending vibration of carboxyl group at 1386 cm−1, the C–OH stretching peak at 1266 cm−1 and the C–O stretching peak at 1044 cm−1. In the spectrum of welan gum, the broad band at 3419 cm−1 corresponds to the hydroxyl groups, the peak at 1727 cm−1 is C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of the carboxylic group of welan gum, and the peaks near 1628 cm−1 and 1452 cm−1 were caused by symmetric and asymmetric stretching vibrations of the –COO groups, respectively.16 For welan gum–GO hybrid hydrogels, it can be seen that both the characteristic peaks of welan gum and GO can be observed and when pH varies, the two peaks between 2700 cm−1 and 2900 cm−1 become more and more obvious due to the increase of COO−, indicating the weakness of hydrogen bonds.17,18
O stretch of the carboxylic group of welan gum, and the peaks near 1628 cm−1 and 1452 cm−1 were caused by symmetric and asymmetric stretching vibrations of the –COO groups, respectively.16 For welan gum–GO hybrid hydrogels, it can be seen that both the characteristic peaks of welan gum and GO can be observed and when pH varies, the two peaks between 2700 cm−1 and 2900 cm−1 become more and more obvious due to the increase of COO−, indicating the weakness of hydrogen bonds.17,18
XRD patterns of the welan gum–GO composite hydrogels further demonstrate that graphene-based sheets are indeed present as individual graphene sheets in the composites. The XRD pattern shows the characteristic peaks of GO around 2θ = 10.6°, which is a clear indication of the structure of GO. The XRD pattern of the welan gum–GO composite hydrogels shows mainly the welan gum diffraction peaks and the diffraction peak of GO nearly disappears (Fig. 3C), which clearly demonstrates the formation of a fully exfoliated structure of GO sheets in the polymer matrix and the disappearance of the regular and periodic structure of GO.19,20 Moreover, it is noticed that the intensity of the characteristic peaks of welan gum obviously increases with the increase of pH (Fig. 3D). It can be deduced that GO plays a role as the rigid template to attach welan gum chains via hydrophobic interaction, electrostatic interaction and hydrogen bonding.
Rheological properties
The rheological properties of the welan gum–GO composite hydrogels with different pH were investigated. From Fig. 4A, we can see that when the sweep frequency is fixed at 1 Hz, G′ is always larger than G′′ within the whole investigated pH range, which indicates that the samples remain elastic. But with the increase of pH, both G′ and G′′ decrease orderly, for example, the maximum value of G′ decreases from 22.3 Pa (pH = 0.8) to 4.828 Pa (pH = 10.6). Correspondingly, G′′ decreases from 8.764 Pa to 2.385 Pa. When the stress is fixed at 1Pa, both G′ and G′′ increase with the increase of sweep frequency, and the system still exhibits an elastic behavior. What's more, the higher the pH, the larger of the change of G′ and G′′ (Fig. 4B). The complex viscosity (η*) decreases with the increase of frequency for these six samples (Fig. 4C) which is typical gel-like behavior. Moreover, steady rheological measurement revealed that the viscosity of hydrogel diminished sharply from 0.01 to 10 s−1 (Fig. 4D), which is characteristic of physically cross-linked self assembled hydrogels. The higher the pH, the lower the viscosity value is. This phenomenon indicates that the intensity of the hydrogel decreases with the increase of pH due to the weakening of hydrogen bonds and increasing of electrostatic repulsion induced by the enhanced carboxyl ionization.21,22Smart swelling properties
In order to study the swelling properties of welan gum–GO composite hydrogels with different pH, a certain amount of freeze-dried hydrogels were immersed in distilled water and weighted to achieve constant weight after 48 h and the results are shown in Fig. 5A. It can be seen that the welan gum–GO composite hydrogels have a better swelling ratio in the alkaline environment instead of the acid environment. Specifically, the swelling ratio of hydrogel is 110% and 300% at pH of 0.8 and 10.6, respectively, which corresponds to an increment of 172%. It turns out that the welan gum–GO composite hydrogel has excellent pH sensitivity. The swelling curve of pH = 0.8 and pH = 10.6 as a function of time is displayed in Fig. 5B. It is seen that the swelling ratio of the welan gum–GO composite hydrogel in the alkaline environment increases faster than that in the acid environment. This phenomenon is intimately related with the ionization degree of carboxyl groups on GO. In the alkaline environment, most of the carboxyl groups are dissociated and generated carboxylate anion, which results in the increase of repulsion force in the network and in turn the swelling ratio. Moreover, the different force of electrostatic interaction between the COOH and COO− groups can also cause a change of the swelling ratio.23What's more, from the SEM results in Fig. 2, it can be seen that the welan gum–GO composite hydrogels have more holes under the alkaline condition than the acid condition, making it easy for water molecules to enter the network. Schematic representation of the network structure of the welan gum–GO hybrid hydrogels at pH = 0.8 and pH = 10.6 are shown in Scheme 2.
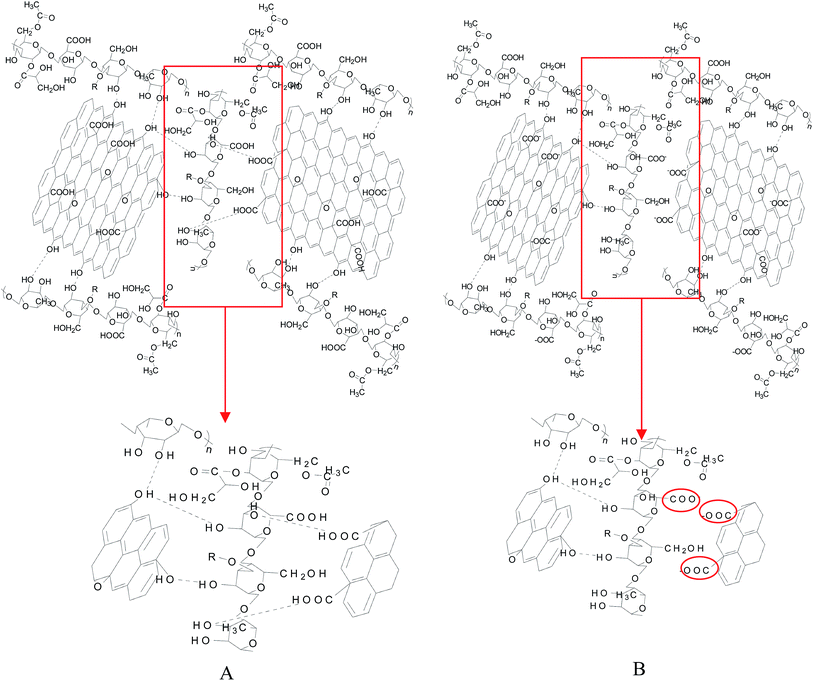 | ||
| Scheme 2 Schematic representation of the network structure of the welan gum–GO hybrid hydrogels: (A) pH = 0.8, (B) pH = 10.6. | ||
The effect of pH on dye adsorption capacity
GO-based composite hydrogels can be prepared by three-dimensional (3D) self-assembly of GO sheets promoted by different types of crosslinking agents.16,23 In these hydrogels, GO sheets remain less aggregated with large surface area, and the interconnected pores in the hydrogels allow adsorbates to diffuse easily into the absorbent. Cheng et al. reported GO–CAS composite hydrogels have large adsorption capacity towards both cationic and anionic dyes as well as metal ions.24,25 As discussed above, the structures of welan gum–GO composite hydrogels can be changed with the variation of pH, so we can infer that pH plays an important role in the whole adsorption process and particularly on the adsorption capacity for dyes. In order to confirm our conjecture, a variety of water soluble dyes such as MB, MV, AB10B, R6G and CAS were used to study the dye adsorption capacity of the welan gum–GO composite hydrogels with different pH. The chemical structures of AB10B, R6G MB, MV, and CAS are provided in Fig. S1.† Fig. 6A and B shows the photographs of the adsorption of different dyes at equilibrium for each sample at pH = 0.8 (Fig. 6A) and pH = 10.6 (Fig. 6B), respectively, while Fig. 6C summarizes the adsorption capacities of the dyes at three different pH. For the anionic dye CAS, it can be seen that the adsorption capacity decreases with increasing pH, which can be ascribed to the increased electrostatic repulsion induced by the increasing number of COO− as mentioned above. For others, the variation of the adsorption capacity at different pH, if there is any, is quite small. As the welan gum–GO composite hydrogels were formed via multiple non-covalent interactions including hydrogen bonds, π–π stacking, and hydrophobic interactions, a change in pH will induce complicated change of these forces.26,27 Thus, the positive change of the adsorption capacity induced by one force may be counteracted by another, accounting for the observed results.Moreover, it can be also found that at pH = 10.6, the hydrogel's adsorption ability for CAS was extremely low (Fig. 6B), so it was taken as a sample to study the change of adsorption under different pH. Photographs of the adsorption for CAS show that when pH value is 10.6, CAS can hardly be absorbed into the hydrogel (Fig. 7A). The concentration of CAS solutions were monitored using UV-vis spectroscopy, which indicates that adsorption capacities on CAS decrease with the increase of pH (Fig. 7B). In addition, we found that when an aqueous solution of CAS mixed together with AB10B was incubated with the gels, selective removal of AB10B at pH = 10.6 was obviously observed, while at pH = 0.8, both of the two dyes were adsorpted completely (Fig. 7C). The selective adsorption experiment confirm the poor adsorption capacity of welan gum–GO composite hydrogel for CAS at high pH.
Adsorption isotherm
Adsorption properties and equilibrium data, commonly known as adsorption isotherms, describe how pollutants interact with sorbent materials and are critical in optimizing the use of adsorbents.15 In order to optimize the design of an adsorption system to remove dye from solutions, it is important to establish the most appropriate correlation for the equilibrium curve. Herein, Langmuir and Freundlich adsorption isotherms are often used to investigate the adsorption process. To further understand the dye adsorption mechanism in our system, various adsorption parameters were calculated by plotting Langmuir and Freundlich isotherm models. The results of the experimental data were described with the Langmuir and Freundlich models. It can be observed from the correlation coefficients that the adsorption data fit the Langmuir isotherm model well, but it can hardly fit the Freundlich one, for which the correlation coefficient is only 0.5242, suggesting the monolayer adsorption of dyes on uniform surface.The Langmuir adsorption isotherm has been successfully applied to many pollutants adsorption process and has been the most widely used adsorption isotherm for the adsorption of solute from a liquid solution, it was deduced by several hypothesis: monolayer adsorption; dynamic balance; no interaction between adsorbate molecules; every adsorption sites have the same adsorptive power.28 The Langmuir equation is obtained as:
 | (3) |
The Langmuir adsorption isotherms for pH = 0.8 and pH = 10.6 are displayed in Fig. 8A, the adsorption capacity calculated from the Langmuir model is 87.5 mg g−1 and 82.5 mg g−1, respectively, confirming that the adsorption capacity for R6G is higher in acidic condition than in alkaline condition.
 | ||
| Fig. 8 (A) Langmuir adsorption isotherm of R6G onto welan gum–GO hydrogels. (B) The pseudo-first-order adsorption model for the adsorption of R6G onto welan gum–GO hydrogels. | ||
Adsorption kinetics
To detect the detailed adsorption process and mechanism of these GO–biopolymer gels, three kinetic models were often used to test the experimental data, i.e. the pseudo-first-order equation, the pseudo-second-order equation and the intraparticle diffusion equation.28,29 To study the reaction kinetics, pseudo-first-order and pseudo-second-order models were used. It can be observed from the correlation coefficients that the adsorption data fit the pseudo-first-order model better than the pseudo-second-order model. The pseudo-first-order kinetic model is more suitable for low concentration of solute. It can be written in the following form:
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (4) |
The pseudo-first-order adsorption model for the adsorption of R6G onto welan gum–GO hydrogels at pH = 0.8 and pH = 10.6 are shown in Fig. 8B, the slopes of the two curves are −0.004 and −0.003 respectively, indicating that the adsorb speed in acidic condition is faster than in alkaline condition.
Conclusions
Biopolymer composite hydrogels were prepared from microbial polysaccharide welan gum as a matrix and GO as a reinforcing additive by a simple self-assembly strategy. The effect of pH on the properties of these composite hydrogels were studied by using phase behavior study, TEM, SEM, FT-IR, XRD and rheological measurements. It can be concluded that the variation of pH could make some changes on structures of hydrogels which can exert an influence on adsorption capacity for dyes. An acidic condition is more beneficial to increase the welan gum–GO composite hydrogel adsorption capacity than an alkaline condition. What's more, with the increase of pH value, the swelling ratio of dry hydrogel also increases, indicating that the welan–GO hydrogel shows greater stability. Thus, we expect that the GO-based composite hydrogels would have potential and more promising applications in the field of waste water treatment by adjusting pH.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (21203109), and Ji'nan Youth Science and Technology Star Program (2013040).References
- J. Fan, L. Li, H. Rao, Q. Yang, J. Zhang, H. Chen, L. Chen, D. Kuang and C. Su, J. Mater. Chem. A, 2014, 37, 15406 Search PubMed.
- C. D. Ampatzidis, E. M. A. Varka and T. D. Karapantsios, Colloids Surf., A, 2014, 460, 176–183 CrossRef CAS.
- M. Rinaudo, Biomacromolecules, 2004, 4, 1155–1165 CrossRef PubMed.
- M. Tako, T. Teruya and Y. Tamaki, Colloid Polym. Sci., 2009, 287, 1445–1454 CAS.
- R. H. Colby, Rheol. Acta, 2010, 49, 425–442 CrossRef CAS.
- A. de Poulpiquet, A. Ciaccafava and E. Lojou, Electrochim. Acta, 2014, 126, 104–114 CrossRef CAS.
- K. M. Liew, Z. X. Lei and L. W. Zhang, Compos. Struct., 2015, 120, 90–97 CrossRef.
- A. A. Maarouf, A. Kasry and B. Chandra, Carbon, 2016, 102, 74–80 CrossRef CAS.
- O. Lv, Y. Tao, Y. Qin, C. Chen, Y. Pan, L. Deng, L. Liu and Y. Kong, Mater. Sci. Eng. C, 2016, 67, 478–485 CrossRef CAS PubMed.
- S. Komathi, N. Muthuchamy, K.-P. Lee and A.-I. Gopalan, Biosens. Bioelectron., 2016, 84, 64–71 CrossRef CAS PubMed.
- Z. Li, J. Shen, H. Ma, X. Lu, M. Shi, N. Li and M. Ye, Soft Matter, 2012, 8, 3139 RSC.
- J. Liu, L. Cui, N. Kong, C. J. Barrow and W. Yang, Eur. Polym. J., 2014, 50, 9–17 CrossRef CAS.
- S. Qin, X. Liu, R. Zhuo and X. Zhang, Macromol. Chem. Phys., 2012, 213, 2044–2051 CrossRef CAS.
- R. Xie, P. Ren, J. Hui, F. Ren, L. Ren and Z. Sun, Carbohydr. Polym., 2016, 138, 222–228 CrossRef CAS PubMed.
- C. Cheng, J. Deng, B. Lei, A. He, X. Zhang, L. Ma, S. Li and C. Zhao, J. Hazard. Mater., 2013, 263, 467–478 CrossRef CAS PubMed.
- M. Yu, A. Song, G. Xu, X. Xin, J. Shen, H. Zhang and Z. Song, RSC Adv., 2015, 92, 75589–75599 RSC.
- H. Bai, C. Li, X. Wang and G. Shi, J. Phys. Chem. C, 2011, 115, 5545–5551 CAS.
- J. Liu, L. Tao, W. Yang, D. Li, C. Boyer, R. Wuhrer, F. Braet and T. P. Davis, Langmuir, 2010, 26, 10068–10075 CrossRef CAS PubMed.
- X. S. Du, M. Xiao, Y. Z. Meng and A. S. Hay, Carbon, 2005, 43, 195–197 CrossRef CAS.
- T. Jiang, Z. Sui, Q. Yang, X. Zhang and B. Han, Soft Matter, 2015, 11, 3215–3221 RSC.
- L. Wang, X. Xin, K. Guo, M. Yang, X. Ma, J. Yuan, J. Shen and S. Yuan, Phys. Chem. Chem. Phys., 2014, 16, 14771–14780 RSC.
- R. Xue, X. Xin, L. Wang, F. Ji, W. Li, C. Jia and G. Xu, Phys. Chem. Chem. Phys., 2015, 17, 5431–5440 RSC.
- H. Chiu, Y. Lin and S. Hung, Macromolecules, 2002, 53, 5235–5242 CrossRef.
- Y. Xu, Q. Wu, Y. Sun, H. Bai and G. Shi, ACS Nano, 2010, 4, 7358–7362 CrossRef CAS PubMed.
- N. Cheng, Q. Hu, Y. Guo, Y. Wang and L. Yu, ACS Appl. Mater. Interfaces, 2015, 7, 10258–10265 CAS.
- T. Tetsumoto, Y. Gotoh and T. Ishiwatari, J. Colloid Interface Sci., 2011, 362, 267–273 CrossRef CAS PubMed.
- J. Sui, L. Wang, W. Zhao and J. Hao, Chem. Commun., 2016, 52, 6993–6996 RSC.
- H. Bai, C. Li, X. Wang and G. Shi, Chem. Commun., 2010, 46, 2376 RSC.
- Y. Yang, Y. Xie, L. Pang, M. Li, X. Song, J. Wen and H. Zhao, Langmuir, 2013, 29, 10727–10736 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18853b |
| This journal is © The Royal Society of Chemistry 2016 |