Copper(II) carboxymethylcellulose (CMC-CuII) as an efficient catalyst for aldehyde–alkyne–amine coupling under solvent-free conditions†
Xiaoping Liu,
Bijin Lin,
Zhuan Zhang,
Hao Lei and
Yiqun Li*
Department of Chemistry, Jinan University, Guangzhou 510632, P. R. China. E-mail: tlyq@jnu.edu.cn
First published on 23rd September 2016
Abstract
A novel and eco-friendly three-component reaction of aldehydes, amines, and alkynes (A3 coupling) catalyzed by a recoverable copper(II) carboxymethylcellulose (CMC-CuII) catalyst has been developed, producing a diverse range of propargylamines under solvent-free conditions in good yields. The CMC-CuII catalyzed reaction is especially effective for reactions involving aromatic and aliphatic aldehydes. High catalytic activity was obtained in the presence of 5 mol% CMC-CuII without any other co-catalyst, additives, or bases under aerobic conditions. Moreover, the catalyst can be easily recovered and reused for at least four cycles without significant decrease in its catalytic activity.
Introduction
Multicomponent reactions (MCRs) combine at least three starting components into a product consisting of a large number of atoms of each starting substrate in a single one-pot process.1 The transition metal catalyzed MCRs are powerful synthetic tools in the synthesis of diverse complex compounds from simple substrates.1b Among the numerous transition-metal catalyzed MCRs, the three component reaction (3CR) of aldehyde, amine, and alkyne (commonly called A3 coupling)2 is one of the well-known examples as it produces valuable N-containing heteroatom propargylamine products. Propargylamines are not only important structural moieties present in natural products and potential pharmaceutical molecules, but also versatile synthetic blocks in various organic transformations.3 Due to their significance, great effort has been made to the synthesis of these compounds. Conventional synthetic protocols for propargylamines include nucleophilic addition of lithium acetylides or Grignard reagents to imines or their derivatives.4 However, in most case, these procedures require the employment of stoichiometric amounts of organometallic reagents under strictly controlled moisture- and air-free conditions. An efficient alternative atom-economical approach is to perform this type of reactions by a catalytic coupling of aldehyde, alkyne, and amine via C–H activation, in which water is the only by-product. The importance of this reaction inspires many chemists to seek new highly active and stable catalysts, mostly based on transition metal species. A variety of transition metal catalysts have been reported to promote this three component reaction. These include transition metal salts or complexes such as Au,5 Ir,6 Ag,7 Fe,8 Ni,9 Zn,10 Co,11 Cu,12 and Cu/Ni,13 Cu/Ru14 bimetallic system under homogeneous reaction conditions. Despite the efficiency of these catalysts, homogeneous catalysts suffer from the drawbacks of recovery and catalytic reuse which severely obstruct their wide use in academic and industrial areas. In order to achieve recyclability of the catalysts, heterogeneous catalysts have received much more attentions recently. Compared to the homogeneous catalysts, the heterogeneous supported catalysts have remarkable characteristics such as easy separation and regeneration, and good recyclability and reusability. Proceed along this line, several heterogeneous catalysts such as supported copper catalysts (Cu-MCM-41, Cu-MS, Cu-HAP, CuI/A-21),15 supported gold catalysts (LDH-AuCl4, Au/CeO2, Au/IRMOF-3),16 supported silver(Ag-G, Ag-K10)17 were successfully employed to catalyze A3-coupling reactions. However, these materials, such as inorganic oxides, polymers, and metal–organic frameworks (MOFs), have at least one drawback such as lacking of active catalytic sites, fragile, non-biodegradable, and are unfriendly to the environment, or their preparation and regeneration is quite tedious.Among the reported catalysts, since copper catalysts are cheap, available, and highly reactive, a lot of copper salts and its complexes as a source of homogeneous or heterogeneous catalyst were successfully employed to catalyze A3-coupling reactions. Recently, several copper heterogeneous catalytic systems were successfully developed to catalyze A3-coupling reactions.15 However, most of these copper heterogeneous catalysts were not easily available, used in toxic solvent or required harsh reaction conditions. As far as we observed, reports on copper heterogeneous catalysts mobilized on biodegradable biopolymer are still very limited and it is desirable to develop highly efficient copper catalysts for A3-coupling reactions under environmentally friendly condition.
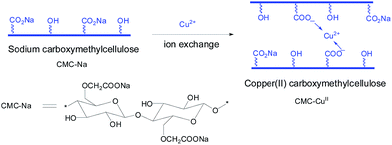
Sodium carboxymethylcellulose (CMC-Na) with carboxymethyl groups (–CH2–COO–Na+) bonded on cellulose backbone is capable of exchanging with metal cations, which makes it an excellent support to immobilize metal catalyst via ion exchange reaction. Very recently, we reported the application of cellulose or carboxymethylcellulose as a support for stabilization of palladium nanoparticles and its applications in Suzuki–Miyaura and Heck–Mizoroki coupling reactions.18 In continuation with our interest on biopolymer-supported heterogeneous catalysts and multicomponent reactions including A3 couplings, herein, we report the synthesis, characterization, and application of copper(II) carboxymethylcellulose (CMC-CuII) as an excellent recyclable catalyst without any additive or base in A3 coupling reactions under solvent-free condition in the air. To the best of our knowledge, so far no application of CMC-CuII catalyst in A3-coupling reaction has been reported in the literature.
Results and discussion
We first describe the preparation and characterization for carboxymethylcellulose-supported Cu(II) (CMC-CuII) and available FT-IR, SEM, EDX, and XPS characterization data to understand the structure of this catalyst before presenting the catalytic activity of CMC-CuII for A3 coupling.Scheme 1 illustrates the preparation procedure to obtain CMC-CuII.
Briefly, CMC-CuII was formed by the metathesis of aqueous copper salts and CMC-Na solution at room temperature. Under this condition, blue solid precipitated from the solution, indicating successful formation of CMC-CuII.
The resulting blue solid catalyst was recovered from the liquid phase by filtration and thoroughly washed with distilled water.
| CMC-Na (aq) + CuSO4 (aq) → CMC-Cu(II)↓ + Na2SO4 (aq) |
The catalyst was finally dried at 60 °C till constant weight. The Cu content was determined to be 2.146 mmol g−1 by ICP.
The characteristic absorption peaks at 1600 and 1420 cm−1 in the FT-IR spectrum of CMC-Na are attributed to asymmetric and symmetric stretching vibration of carboxylate (–COO−) group, respectively. In the IR spectrum of CMC-CuII, these peaks shifted to 1631.2 and 1427.5 cm−1, implying coordination of –COO− with CuII (Fig. 1).
SEM images of CMC-CuII showed that the catalyst was made up of micro-particles with an average diameter of around 100 μm (Fig. 2a). Also, the presence of carbon, oxygen and copper in the CMC-CuII was confirmed by energy-dispersive X-ray spectroscopy (EDX) (Fig. 2b) associated with SEM analysis. As a result, it is reasonable to assume that CMC molecules can serve as an effective matrix to bond Cu(II) species.
XPS was performed to elucidate the valence state of Cu on the surface region of the catalyst. As shown in Fig. 3, the binding energies of Cu2p in the fresh (Fig. 3a) and recovered CMC-CuII (Fig. 3b) are measured to be 933.6 eV. These results indicated that the presence of Cu(II) species in both freshly prepared and recovered CMC-CuII catalyst.
We used benzaldehyde (1a), morpholine (2a) and phenylacetylene (3a) as standard substrates to search for the optimal condition for the A3-coupling. Initially, various simple metal salts such as FeCl3, CuSO4, Cu(OAc)2, CuI and CMC-CuII were investigated for the coupling reaction (Table 1). The presence of metal catalyst proved to be crucial for the A3 coupling. No desired product was detected when the reaction was carried out in the absence of catalyst (Table 1, entry 5). In the control experiment, CMC-Na could not promote the reaction effectively (Table 1, entry 6). Among all the catalysts evaluated, CMC-CuII was found to be most effective in catalyzing the three component coupling. Next, various solvents were explored for the coupling reaction and higher yields were observed when nonpolar solvent was used (Table 1, entries 7–12). In addition, it is noteworthy that the reaction proceeded far more efficiently under neat condition (Table 1, entries 13–18).
| Entry | Catalyst (mol%) | Solvent | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), morpholine (1.2 mmol), phenylacetylene (1.5 mmol) in the air.b Isolated yields.c No product. | |||||
| 1 | FeCl3 (10) | — | 100 | 15 | 55 |
| 2 | CuSO4 (10) | — | 100 | 15 | 25 |
| 3 | Cu(OAc)2 (10) | — | 100 | 15 | 59 |
| 4 | CuI (10) | — | 100 | 15 | 79 |
| 5 | — | — | 100 | 15 | NPc |
| 6 | CMC-Na (10) | — | 100 | 15 | 9 |
| 7 | CMC-CuII (5) | H2O | 100 | 12 | 37 |
| 8 | CMC-CuII (5) | EtOH | 80 | 15 | 34 |
| 9 | CMC-CuII (5) | CH3CN | 80 | 15 | 28 |
| 10 | CMC-CuII (5) | PEG-400 | 100 | 15 | 56 |
| 11 | CMC-CuII (5) | Toluene | 90 | 15 | 73 |
| 12 | CMC-CuII (5) | Toluene | 100 | 15 | 79 |
| 13 | CMC-CuII (4) | — | 100 | 15 | 73 |
| 14 | CMC-CuII (5) | — | 100 | 15 | 81 |
| 15 | CMC-CuII (10) | — | 100 | 15 | 74 |
| 16 | CMC-CuII (5) | — | 80 | 15 | 65 |
| 17 | CMC-CuII (5) | — | 90 | 15 | 72 |
| 18 | CMC-CuII (5) | — | 100 | 12 | 72 |
It is also found that the catalyst loading greatly affects the yield of the coupling products. By increasing the amount of the CMC-CuII catalyst from 4, 5 to 10 mol%, the yield reached up to 73, 81, and 74% respectively (Table 1, entries 13–15). Thus, 5 mol% of catalyst is believed to be enough to complete the reaction. As evident from Table 1, the reaction temperature has a significant effect on the coupling reaction. It was also seen that decreasing the temperature from 100 to 80 °C provided lower yields (Table 1, entries 15 and 16). Thus, the results revealed that the best reaction condition for this catalytic process includes the usage of 1.0 equiv. of aldehyde, 1.2 equiv. of amine, 1.5 equiv. of alkyne, together with 5 mol% of CMC-CuII under neat conditions at 100 °C in the air (Table 1, entry 14).
With the optimized reaction conditions in hand, various aldehydes, amines, and alkynes were screened to explore the scope of the three-component coupling reaction. The detailed results were summarized in Table 2. Aryl aldehydes with both electron-donating and electron-withdrawing functionalities afforded the corresponding propargylamines in good to excellent yields. In most cases, aryl aldehydes with electron-withdrawing groups led to slightly higher yields compared to those with electron-donating groups, when morpholine and phenylacetylene were employed (Table 2, entries 2–10). However, with aryl aldehydes carrying strong electron-releasing groups (i.e. –N(CH3) or –OH), only trace amount of desired product was isolated (Table 2, entries 11 and 12). When aliphatic aldehydes reacted with morpholine and phenylacetylene, formaldehyde is more active than others, afforded the desired product in 80% yield (Table 2, entry 13).
| Entry | Aldehydes (1) | Amines (2) | Alkynes (3) | Products (4) | Yieldsb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: aldehyde (1.0 mmol), amine (1.2 mmol), alkyne (1.5 mmol), CMC-Cu (5 mol%), 100 °C, 15 h, under solvent-free in the air.b Isolated yields. | |||||
| 1 |  |
 |
 |
 |
81 |
| 2 |  |
 |
 |
 |
85 |
| 3 |  |
 |
 |
 |
86 |
| 4 |  |
 |
 |
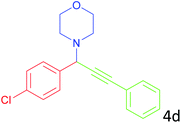 |
88 |
| 5 |  |
 |
 |
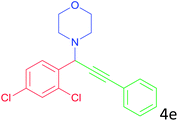 |
78 |
| 6 |  |
 |
 |
 |
85 |
| 7 |  |
 |
 |
 |
84 |
| 8 |  |
 |
 |
 |
82 |
| 9 |  |
 |
 |
 |
80 |
| 10 |  |
 |
 |
 |
83 |
| 11 |  |
 |
 |
 |
Trace |
| 12 |  |
 |
 |
 |
Trace |
| 13 |  |
 |
 |
 |
80 |
| 14 |  |
 |
 |
 |
60 |
| 15 |  |
 |
 |
 |
55 |
| 16 |  |
 |
 |
 |
80 |
| 17 |  |
 |
 |
 |
79 |
| 18 |  |
 |
 |
 |
88 |
| 19 |  |
 |
 |
 |
78 |
| 20 |  |
 |
 |
 |
Trace |
| 21 |  |
 |
 |
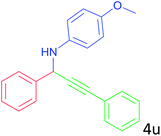 |
20 |
| 22 | 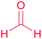 |
 |
 |
 |
21 |
| 23 |  |
 |
 |
 |
85 |
| 24 |  |
 |
 |
 |
82 |
| 25 |  |
 |
 |
 |
43 |
| 26 |  |
 |
 |
 |
83 |
Different amines also underwent the corresponding three-component coupling reaction smoothly and generated the products in good to excellent yields. Moderate to good yields were observed when cyclic dialkylamines such as morpholine, piperidine, and pyrrolidine (Table 2, entries 1–17, and 23–26) or secondary aromatic amines (Table 2, entries 18 and 19) were used. By replacing the secondary amine with primary aromatic amine (such as aniline), a significant decrease in the reaction yield was observed (Table 2, entries 20–22), owing to the stereoelectronic effects making the nucleophilic attack of the nitrogen atom of the amine more difficult in forming the intermediate imine. Once again, aliphatic alkyne leads to lower yield (Table 2, entry 25). Presumably, the aliphatic alkyne is not as effective in forming a Cu-alkyne intermediate when compared to aromatic alkynes.
Proposed mechanism for the reaction is based on the reported metal-catalyzed A3-coupling reaction mechanism (Scheme 2). It is suggested that the A3 coupling proceeds initially by activation of alkyne by Cu(II) (3A). After deprotonation, the alkyl-Cu intermediate was coordinated with the iminium ion (2A) generated in situ from the aldehyde and amine to form the activated complex (4A), which was further nucleophilic attacked by alkyl-Cu to produce the corresponding propargylamine (4) and regenerate the Cu(II) catalyst for further cycling.
For practical applications of heterogeneous catalyst, the reusability of catalyst is a very important factor.
For the study of recycling abilities of this CMC-CuII catalyst, the model reaction of 3-methoxybenzaldehyde, dibenzylamine and phenylacetylene under the optimal reaction condition was investigated (Scheme 3). Results indicated that this catalyst is recyclable during at least four consecutive runs with only slight decreasing in its catalytic activity. In addition, the TON (turnover number) and TOF (turnover frequency) of this reaction, were calculated to be 18 and 2.1 h−1, respectively. Furthermore, to determine the nature of the catalysis, we have also performed the hot filtration test by using the model reaction under the optimal reaction condition. The reaction was terminated in 1 h (∼32% conversion) by adding excessively hot ethanol to filter out the solid catalyst. After removal of ethanol, the reaction was then allowed to react for another 12 h at 100 °C under solvent-free condition. After this time, no further progress in product yield was observed. The results revealed that essentially no Cu(II) species was leached out into the reaction mixture and the reaction has truly been catalyzed by a heterogeneous system.
 | ||
| Scheme 3 Recycling of catalyst for the reaction of 3-methoxybenzaldehyde, dibenzylamine and phenylacetylene. | ||
Experimental
FT-IR spectra were recorded on a Nicolet 6700 spectrometer as KBr pellets in the range 4000–400 cm−1. 1H NMR (300 MHz) and 13C NMR (75 MHz) spectra were obtained with a Bruker Avance instrument with CDCl3 as solvent and TMS as internal standard. HRMS data were recorded using AB SCIEX Triple TOF 5600+ detection. The elemental copper content of polymeric catalysts was determined by PerkinElmer Optima 2000DV inductively coupled plasma-atomic emission spectrometry (ICP-AES). X-ray photoelectron spectroscopy (XPS) measurements were performed on a KratosAxis UltraDLD X-ray photoelectron spectrometer with an Al Kα excitation. Scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX) were performed with JEM-2100F instrument. All chemicals were obtained from commercial sources and used as received.Preparation of copper(II) carboxymethylcellulose (CMC-CuII)
The 1 wt% aqueous solution of sodium carboxymethylcellulose was added dropwise to an aqueous solution containing 10 wt% CuSO4·5H2O with constantly stirring at room temperature. The blue solid was precipitated immediately and further left equilibrate in solution for 6 h. The resulting solid was separated from the solution by suction filtration and washed thoroughly with distilled water, then dried in vacuum to constant weight to provide the CMC-CuII as blue powder. The Cu content was determined to be 2.146 mmol g−1 by ICP-AES.General procedure for A3-coupling reaction
A mixture of aldehyde (1.0 mmol), amine (1.2 mmol) and phenylacetylene (1.5 mmol) and a catalytic amount of CMC-CuII (5 mol%) was stirred in neat at 100 °C in air for 15 h. After the completion of reaction, 30 mL EtOAc was added to dissolve the product. The catalyst was separated by filtration and the filtrate was washed with brine and dried over anhydrous Na2SO4. The product was obtained after removal of the organic fractions using a rotary evaporator. The desired pure products were further purified by preparative TLC. All the products except 4i, 4r, 4s, 4w, 4x, 4y and 4z are known, and their 1H NMR data were found to be identical to those reported in the literature. The new compounds were fully characterized by FT-IR, 1H NMR, 13C NMR, MS, and HR-MS.Conclusions
In conclusion, a new carboxymethylcellulose-supported Cu(II) catalyst has been synthesized, characterized, and used as an inexpensive, renewable and biodegradable catalyst for three-component coupling reaction of amines, aldehydes, and phenylacetylene. A variety of aldehydes, amines and alkynes proceed smoothly in the absence of solvent, any co-catalyst, other additive, and base under aerobic conditions and excellent yields were obtained. The catalyst was recycled for four consecutive runs. The CMC-CuII catalyst can be regarded as an environmentally benign catalyst, which is derived from the advantages of cellulosic derivatives and the high catalytic activity in A3 couplings, as well as reusability.Acknowledgements
We are grateful to the National Natural Science Foundation of China (No. 21372099 and 21301070), the Guangdong Natural Science Foundation (No. 10151063201000051 and 8151063201000016) for financial support.Notes and references
- For selected examples, see: (a) B. Ganem, Acc. Chem. Res., 2009, 42, 463–472 CrossRef CAS PubMed; (b) D. M. D'Souza and T. J. J. Müller, Chem. Soc. Rev., 2007, 36, 1095–1108 RSC; (c) M. D. Burke and S. L. Schreiber, Angew. Chem., Int. Ed., 2004, 43, 46–58 CrossRef PubMed; (d) L. Weber, Drug Discovery Today, 2002, 7, 143–147 CrossRef CAS PubMed.
- (a) V. A. Peshkov, O. P. Pereshivko and E. V. Van der Eycken, Chem. Soc. Rev., 2012, 41, 3790–3807 RSC; (b) W. Yoo, L. Zhao and C. Li, Aldrichimica Acta, 2011, 44, 43–51 CAS.
- (a) A. A. Boulton, B. A. Davis, D. A. Durden, L. E. Dyck, A. V. Juorio, X. M. Li, I. A. Paterson and P. H. Yu, Drug Dev. Res., 1997, 42, 150–156 CrossRef CAS; (b) L. Zani and C. Bolm, Chem. Commun., 2006, 4263–4275 RSC; (c) A. Ranjan, R. Yerande, P. B. Wakchaure, S. G. Yerande and D. H. Dethe, Org. Lett., 2014, 16, 5788–5791 CrossRef CAS PubMed.
- (a) R. Díez, R. Badorrey, M. D. Díaz-de-Villegas and J. A. Gálvez, Eur. J. Org. Chem., 2007, 2114–2120 CrossRef; (b) K. B. Aubrecht, M. D. Winemiller and D. B. Collum, J. Am. Chem. Soc., 2000, 122, 11084–11089 CrossRef CAS; (c) T. Murai, Y. Mutoh, Y. Ohta and M. Murakami, J. Am. Chem. Soc., 2004, 126, 5968–5969 CrossRef CAS PubMed.
- (a) C. Wei and C.-J. Li, J. Am. Chem. Soc., 2003, 125, 9584–9585 CrossRef CAS PubMed; (b) V. Srinivas and M. Koketsu, Tetrahedron, 2013, 69, 8025–8033 CrossRef CAS; (c) V. K. Y. Lo, Y. Liu, M. K. Wong and C. M. Che, Org. Lett., 2006, 8, 1529–1532 CrossRef CAS PubMed.
- (a) Y. Zhang, P. Li, M. Wang and L. Wang, J. Org. Chem., 2009, 74, 4364–4367 CrossRef CAS PubMed; (b) J. S. Yadav, B. V. Subba Reddy, A. V. Hara Gopal and K. S. Patil, Tetrahedron Lett., 2009, 50, 3493–3496 CrossRef CAS.
- (a) C. Wei, Z. Li and C. J. Li, Org. Lett., 2003, 5, 4473–4475 CrossRef CAS PubMed; (b) M. Trose, M. Dell'Acqua, T. Pedrazzini, V. Pirovano, E. Gallo, E. Rossi, A. Caselli and G. Abbiati, J. Org. Chem., 2014, 79, 7311–7320 CrossRef CAS PubMed; (c) O. Prakash, H. Joshi, U. Kumar, A. K. Sharma and A. K. Singh, Dalton Trans., 2015, 44, 1962–1968 RSC.
- (a) P. Li, Y. Zhang and L. Wan, Chem.–Eur. J., 2009, 15, 2045–2049 CrossRef CAS PubMed; (b) W. W. Chen, R. V. Nguyen and C. J. Li, Tetrahedron Lett., 2009, 50, 2895–2898 CrossRef CAS.
- S. Samai, G. C. Nandi and M. S. Singh, Tetrahedron Lett., 2010, 51, 5555–5558 CrossRef CAS.
- (a) M. Periasamy, P. O. Reddy, A. Edukondalu, M. Dalai, L. M. Alakonda and B. Udaykumar, Eur. J. Org. Chem., 2014, 6067–6076 CrossRef CAS; (b) E. Ramu, R. Varala, N. Sreelatha and S. R. Adapa, Tetrahedron Lett., 2007, 48, 7184–7190 CrossRef CAS.
- W.-W. Chen, H.-P. Bi and C.-J. Li, Synlett, 2010, 475–479 CAS.
- (a) H.-B. Chen, Y. Zhao and Y. Liao, RSC Adv., 2015, 5, 37737–37741 RSC; (b) T. T. T. Trang, D. S. Ermolat'ev and E. V. Van der Eycken, RSC Adv., 2015, 5, 28921–28924 RSC; (c) U. C. Rajesh, G. Purohit and D. S. Rawat, ACS Sustainable Chem. Eng., 2015, 3, 2397–2404 CrossRef CAS.
- S. V. Katkar and R. V. Jayaram, RSC Adv., 2014, 4, 47958–47964 RSC.
- E. Ryan Bonfield and C.-J. Li, Org. Biomol. Chem., 2007, 5, 435–437 Search PubMed.
- (a) M. Abdollahi-Alibeik and A. Moaddeli, RSC Adv., 2014, 4, 39759–39766 RSC; (b) M. Gholinejad, F. Saadati, S. Shaybanizadeh and B. Pullithadathil, RSC Adv., 2016, 6, 4983–4991 RSC; (c) B. M. Choudary, C. Sridhar, M. L. Kantam and B. Sreedhar, Tetrahedron Lett., 2004, 45, 7319–7321 CrossRef CAS; (d) G. Bosica and J. Gabarretta, RSC Adv., 2015, 5, 46074–46087 RSC.
- (a) X. Zhang and A. Corma, Angew. Chem., Int. Ed., 2008, 47, 4358–4361 CrossRef CAS PubMed; (b) L. He, Y. Liu, J. Liu, Y. Xiong, J. Zheng, Y. Liu and Z. Tang, Angew. Chem., Int. Ed., 2013, 52, 3741–3745 CrossRef CAS PubMed; (c) M. L. Kantam, B. V. Prakash, C. R. V. Reddy and B. Sreedhar, Synlett, 2005, 2329–2332 CrossRef CAS.
- (a) N. Salam, A. Sinha, A. Singha Roy, P. Mondal, N. R. Jana and S. M. Islam, RSC Adv., 2014, 4, 10001–10012 RSC; (b) M. Jeganathan, A. Dhakshinamoorthy and K. Pitchumani, ACS Sustainable Chem. Eng., 2014, 2, 781–787 CrossRef CAS.
- (a) J. Xiao, Z. Lu and Y. Li, Ind. Eng. Chem. Res., 2015, 54, 790–797 CrossRef CAS; (b) J. Xiao, Z. Lu, Z. Li and Y. Li, Appl. Organomet. Chem., 2015, 29, 646–652 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization data. See DOI: 10.1039/c6ra18742k |
| This journal is © The Royal Society of Chemistry 2016 |