Concentration-induced structural transition of block polymer self-assemblies on a nanoparticle surface: computer simulation†
Abstract
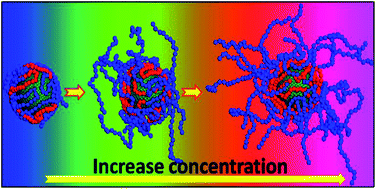
Coarse-grained molecular dynamic simulation (CGMD) techniques are used to study the self-assembly behavior of Pluronic triblock copolymers on different hydrophobicity nanoparticle (NP) surfaces. The structural changeover as a function of concentration of Pluronic triblock copolymers is investigated. At lower concentration (e.g. 5%), a shell-layered film structure self-assembled on NP surfaces is observed. Above 5%, the stretching growth of polymer chains takes place during the adsorption process and the star-shaped film on NP surfaces is formed. As the simulation results show, the radius of gyration of F127 assembled on the NPs would increase with increase of polymer concentration. A transition from incomplete to complete stretching polymer chain structure growth in solvent is encountered with increasing concentrations, which appears as a high phase separation film in terms of order parameter. These changes of the self-assembled films are not majorly initiated by the hydrophobic degrees of NP surfaces, but originate from the domination of polymer concentration.

 Please wait while we load your content...
Please wait while we load your content...