DOI:
10.1039/C6RA18736F
(Paper)
RSC Adv., 2016,
6, 111120-111128
Synthesis, adsorption kinetics and thermodynamics of ureido-functionalized Pb(II) surface imprinted polymers for selective removal of Pb(II) in wastewater
Received
25th July 2016
, Accepted 13th October 2016
First published on 20th October 2016
Abstract
A novel ureido-functionalized Pb(II)-imprinted polymer (Pb(II)-IIP) was synthesized using Pb(II) ion, γ-ureidopropyltrimethoxysilane, AIBN and EGDMA as template ion, functional monomer, initiator and cross-linking agent, respectively. Pb(II)-IIP was characterized by FTIR and SEM. The effect of initial concentration of Pb(II) ions, adsorption time, temperature and pH values on the adsorption capacity of Pb(II)-IIP were studied. The equilibrium adsorption capacity of Pb(II)-IIP could reach 97.5 mg g−1. Langmuir models were adopted and R2 was 0.9908. The adsorption process is endothermic and could occur spontaneously. The relative selectivity coefficients of Pb(II)-IIP were 3.55, 7.02, 3.77 for Pb2+/Cd2+, Pb2+/Cu2+, Pb2+/Zn2+ respectively.
1. Introduction
It is well known that lead is a toxic heavy metal, which does great harm to the human body. Therefore, developing reliable methods to remove lead is becoming increasingly important. Currently, the removal methods include adsorption, ion exchange, separation, chemical precipitation, etc. Among these methods, adsorption is an effective technology. Ion imprinted polymers have gained much attention because of their advantages of large adsorption and specific binding to target ions.1
Ion imprinted polymers (IIPs) are versatile for the removal of heavy metals from waste water. The preparation methods for IIPs include bulk polymerization, suspension polymerization, precipitation polymerization, emulsion polymerization and surface imprinting. Among these methods, the most effective technology is surface imprinting method. It has become more and more important in separation,2 adsorption3 and solid phase extraction.4 IIP could be grafted on the surface of the supporting materials, such as silica gel, alumina, capillary, carbon nanotube, chitosan, etc.,5 to synthesize surface ion imprinted polymers.6 And these imprinting materials not only have the function of molecular/ion imprinting, but also possess good mechanical and thermal stability, high adsorption selectivity7,8 and good mono-dispersity.
Surface ion imprinted polymer have the advantage of high selectivity towards template ions, more imprinted sites, faster binding kinetics, etc.9 Wang10 successfully synthesized a surface Pb(II)-imprinted silica polymer using hydroxamic acids as ion chelating agent and the adsorption capacity is 56.2 mg g−1. Liu11 synthesized a Pb(II) surface imprinted mesoporous polymer and the adsorption capacity reached 36.56 mg g−1. Ghoohestani12 synthesized Pb(II) ion-imprinted polymer on the surface of MCM-41, the adsorption capacity is only 57.7 mg g−1 under the optimum conditions. Jiang13 used diatomite as core material and epichlorohydrin as the cross-linking agent to synthesize a Pb(II)-imprinting polymer and the maximum adsorption capacity reached to 139.6 mg g−1 (the initial concentration of Pb2+ = 600 mg L−1), but the adsorption equilibrium time was up to 3 h. In addition, the surface imprinting technique can also be combined with other technologies to synthesize novel Pb(II) ion-imprinted polymer. Tong14 combined the surface imprinting technique with electro-spinning to synthesize Pb(II) ion-imprinted chelating nano fibers. Wang15 combined the surface imprinting technique with a sol–gel process to prepare a Pb(II) ion-imprinted mesoporous polymer and the adsorption capacity was 221 mg g−1, the relative selectivity coefficient for Cu2+ was only 1.9. Fan16 employed a bidentate chelating ligand to prepare Pb(II)-imprinted silica-supported organic–inorganic hybrid polymer by coupling a surface imprinting technique with sol–gel technology, the adsorption capacity reached quickly 54.9 mg g−1 within 30 min.
However, there is no report about the synthesis of ureido-functionalized Pb(II) ion-imprinted polymer (Pb-IIP) by surface imprinting technique. In this study, ureido-functionalized Pb(II) surface imprinted polymer was synthesized onto the surface of silica gel. Pb(II) ion, γ-ureidopropyltrimethoxysilane, 2,2-azobisisobutyronitrile (AIBN) and ethyleneglycol dimethacrylate (EGDMA) was used as the template ion, functional monomer and cross-linking agent. Pb(II)-IIP was characterized by using scanning electron microscope (SEM) and Fourier transform infrared spectrometry (FTIR). The influence of adsorption time, temperature and pH value on the adsorption kinetics and thermodynamics of the ureido-functionalized Pb(II)-imprinted polymer was investigated.
2. Materials and methods
2.1 Chemicals and reagents
Methanol, ethanol, silica gel (100–200 mesh), γ-ureidopropyltrimethoxysilane, CuCl2·2H2O, CdCl2·2.5H2O and Zn(NO3)2·6H2O were purchased from Sinopharm Chemical Reagent Co., Ltd., China. Lead nitrate (Pb(NO3)2) was supplied by Tianjin Damao Chemical Reagent Industry, China. Ethylene glycol dimethacrylate (EGDMA) was obtained from ACROS Organics (New Jersey, USA.). 2,2-Azobisisobutyronitrile (AIBN) was from Shanghai Four Hervey Chemical Co., Ltd., China. All of these chemicals were of analytical reagent grade. Double distilled water (DDW), used in the experiments, was prepared by Milli-Q advantage purification system (Merck Millipore Ltd., France).
2.2 Instruments and analytical methods
The measurement of lead was carried out with a TAS-990F flame atomic adsorption spectrometer (FAAS) (Beijing Purkinje General Instrument Co., Ltd., China). pH values were measured by a pHS-3C digital pH meter (Shanghai Precision & Scientific Instrument Co., Ltd., China). JSM-6700F Field Emission Scanning Electronic Microscope (Japan Electron Optics Laboratory Co., Ltd., Japan) were used to analyze the morphological characteristics of IIP. Fourier transform infrared spectra (FT-IR, 4500–500 cm−1) (Bruker, Germany) with KBr pellets was recorded by Bruker Tensor 27 FT-IR spectrometer.
2.3 Synthesis of Pb(II)-IIP
Silica gel was activated with 3 mol L−1 hydrochloric acid (HCl) and stirred continuously and constantly for 24 h at 323.15 K. The solution was repeatedly washed with distilled water until the filtrate was neutral. Then the activated silica gel was dried in a vacuum oven.
2 g Pb(NO3)2 was dissolved in methanol, the solution was stirred for 1 h at 323.15 K. Then, 5.35 g γ-ureidopropyltrimethoxysilane dissolved in methanol was added to the solution. The resulting solution was stirred continuously for 1 h, followed by the addition of 3.0 g of activated silica gel. After 8 h of stirring, 0.17 g AIBN as initiator dissolved in methanol and EGDMA as cross-linking agent was slowly added to the solution. The resulting solution was continuously stirred for another 8 h. Finally, the product was washed several times with ethanol and water until it reached neutralization. After drying, the product was treated with 6 mol L−1 HCl to completely remove the unreacted Pb(II). At last, the Pb(II) imprinted polymer was filtered with distilled water and was dried at 323.15 K in a vacuum. In order to compare with Pb(II)-IIP, non-imprinted polymer (NIP) was prepared by following the same method, but without using any Pb(NO3)2.
2.4 Adsorption experiments
2.4.1 Batch experiments for Pb(II) adsorption. Batch experiments were carried out in closed conical flasks to investigate the properties of the imprinted material. The effects of initial concentration, reaction time, temperature and pH values were studied in this paper. The pH values of the solutions were adjusted by using dilute HCl or NaOH. Typically, for each experiment, 10 mg of Pb(II)-IIP was added into 50.0 mL flask containing a certain concentration of Pb(II) at a pH value of 5. Then the mixture was shaken vigorously for 70 min. After filtration, Pb2+ concentration in the supernatant was determined by FAAS. Equilibrium adsorption capacity Qe (mg g−1) and adsorption amount Qt (mg g−1) at time t (min) were calculated by using formulas (1) and (2).| |
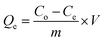 | (1) |
| |
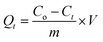 | (2) |
where Co (mg L−1) represents the initial concentrations of Pb(II), Ce (mg L−1) represents the equilibrium concentrations of Pb(II), Ct (mg L−1) represents the time t (min) respectively, V (L) represents the volume of solution and m (g) is the mass of Pb(II) imprinted polymer.
2.4.2 Selectivity experiments. Competitive adsorption of Pb2+/X (X: other metal ions) was carried out to study the selective adsorption behavior of Pb(II) on IIP and NIP. In a typical experiment, 10 mg of IIP and NIP was added into binary mixture solutions of Pb(II)/Cu(II), Pb(II)/Cd(II), and Pb(II)/Zn(II) with initial concentrations of 100 mg L−1, which was due to the fact that they have the same charge and similar ionic radii. The resultant solutions were shaken at a pH of 4 for 70 min. The distribution coefficient kd (mL g−1), selectivity coefficient k, and the relative selectivity coefficient k′ were calculated according to the formulas (3)–(5).17| |
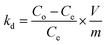 | (3) |
| |
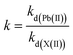 | (4) |
| |
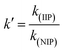 | (5) |
where all the terms used carry the same meanings as what have previously (in eqn (1) and (2) and preceding paragraph) been explained.
3. Results and discussion
3.1 Synthesis and characterization of IIP
3.1.1 Synthesis of IIP. The scheme for synthesis of IIP has clearly been shown in Fig. 1. During the synthesis process of Pb(II)-IIP, Pb(II) ion, γ-ureidopropyltrimethoxysilane, 2,2-azobisisobutyronitrile (AIBN) and ethyleneglycol dimethacrylate (EGDMA) was used as template ion, functional monomer, initiator and cross-linking agent, respectively. Silica gel is a highly active support material containing siloxane (Si–O–Si) and silanol (Si–OH) groups. The silanol groups on the surface of silica gel were chemically modified by introducing ureido groups with γ-ureidopropyltrimethoxysilane into Pb(II)-IIP at 323.15 K. Then ureido-functionalized silica gel particles undergo the polymerization reaction with template ion (Pb(II)) by EGDMA and AIBN. After the elution of the imprinted lead ions, there are some binding sites matched with lead ions on the surface of IIP, thus forming Pb(II)-IIP.
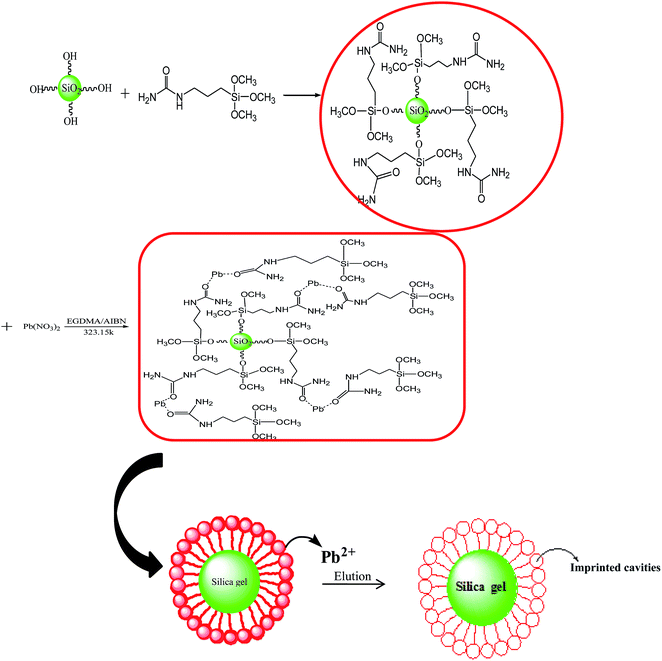 |
| | Fig. 1 The preparation of Pb(II)-imprinted polymer. | |
3.1.2 Characterization of IIP by SEM. In this study, the surface morphology of Pb(II)-IIP was characterized by SEM with desired magnifications and a scanning voltage of 10 kV and results have been shown in Fig. 2. It could be seen that there is a clear difference in the surfaces of Pb(II)-IIP (see Fig. 2a) and NIP (see Fig. 2b). The surface of Pb(II)-IIP is rough with lots of holes belonging to the active sites after elution, which indicates that the template ions have been imprinted within IIP. The imprinted ions cover these holes in the surface, resulting in imprinted cavities on the surface of Pb(II)-IIP. In terms of the differences of Pb(II)-IIP and NIP, it could be concluded that Pb(II) ions played an important role in the synthesis of ion imprinted polymer.
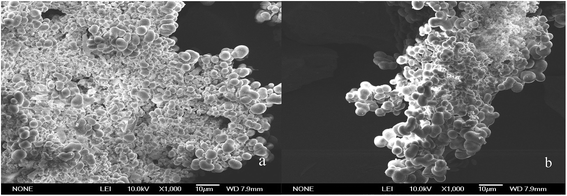 |
| | Fig. 2 SEM images of IIP and NIP. (a) IIP and (b) NIP. | |
3.1.3 FT-IR spectra of IIP. In order to verify the presence of various functional groups on Pb(II)-IIP and NIP, FT-IR spectra were recorded and shown in Fig. 3. From Fig. 3, the spectrum of Pb(II)-IIP was similar to that of NIPs at the same wave number. The presence of –NH2 is reflected by the characteristic bands of 3500 cm−1 and 3400 cm−1, which correspond to asymmetric stretching vibration and symmetric stretching vibration of –NH2 respectively. However, the characteristic bands of 1525 cm−1 was due to the stretching vibration of associating state of –NH2. Due to the presence of –OCH3 and –CH2NH–, an adsorption band of moderate intensity emerged in the range of 2850–2700 cm−1. An intense adsorption band appeared at 1090 cm−1 owning to the siloxane stretching vibrations of (SiO)n. In addition, a peak is noted at 1728 cm−1 due to the carbonyl groups from EGDMA. These results suggested that γ-ureidopropyltrimethoxysilane has successfully been grafted onto the surface of silica gel.
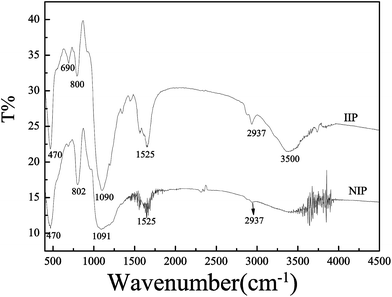 |
| | Fig. 3 FT-IR spectra of IIP and NIP. | |
3.2 Effects of different conditions on the adsorption capacity
3.2.1 Effect of initial concentration of Pb(II) on the adsorption capacity of Pb(II)-IIP. The dosage of Pb(II)-IIP is an important parameter because it determines the adsorption capacity of Pb(II)-IIP. In this work, the dosage of Pb(II)-IIP is 10 mg. The adsorption capacity of Pb(II)-IIP and NIP with respect to different initial concentrations of Pb(II) ions are shown in Fig. 4. The equilibrium adsorption amount increased with the concentration of Pb(II) from 6 mg L−1 to 100 mg L−1. However, when the concentration further increased from 100 mg L−1 to 180 mg L−1, the amount of adsorption tended to balance and reached a constant value of 97.5 mg g−1. The reason is that as the concentration of lead in the solution increases, Pb(II) ion can accumulate on the surface of Pb(II)-IIP and contribute to the effective adsorption of Pb(II) ions, leading to the increasing of the adsorption amount of Pb(II)-IIP. For a Pb(II) concentration of 100 mg L−1, the adsorption amount of Pb(II)-IIP reaches the maximum and remains unchanged. Therefore, the functional groups and the binding sites on Pb(II)-IIP had been completely occupied, due to the saturation of the adsorption capacity of Pb(II)-IIP. Fig. 4 clearly shows that the maximum adsorption capacities of Pb(II)-IIP and NIP for Pb(II) were 97.5 mg g−1 and 31.2 mg g−1 at a Pb(II) concentration of 100 mg L−1. Compared to NIP, the maximum adsorption capacity of Pb(II)-IIP is higher than that of NIP, which is most probably due to the imprinted sites created in the Pb(II)-IIP, which are complementary to Pb(II) ions due to their shape, size and functional groups. However, other ions could not enter the imprinted cavities, so the concentration of lead ions used in subsequent experiments was 100 mg L−1.
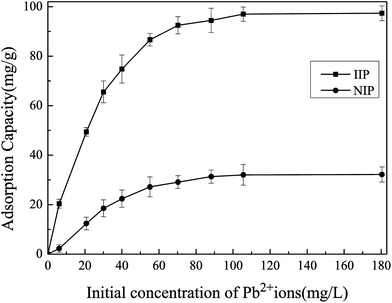 |
| | Fig. 4 Adsorption capacity of IIP and NIP adsorbents for Pb(II). Conditions: pH = 4.0, time = 60 min, temperature = 303.15 K, ion-imprinted adsorbent = 10 mg. | |
3.2.2 Effect of reaction time on the adsorption capacity of Pb(II)-IIP. The effect of reaction time on the adsorption of Pb(II) was presented in Fig. 5. From Fig. 5, the adsorption capacity of Pb(II)-IIP increases constantly as the adsorption time increases to 40 min, and then the amount of adsorption increases slowly. When the adsorption time is 60 min and the saturation of the adsorption capacity is 97.5 mg g−1, the adsorption equilibrium is reached. Results suggested the adsorption rate of Pb(II)-IIP is relatively fast and the equilibration time is short, which was most probably due to the fact that there were a large number of adsorption sites on the surface of Pb(II)-IIP. Pb2+ can bind with the imprinted cavities on the surface of Pb(II)-IIP. Therefore, an adsorption time of 60 min was chosen for further experiments.
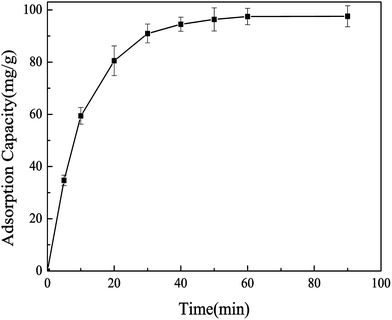 |
| | Fig. 5 Effect of reaction time on the adsorption equilibrium for Pb(II). Conditions: initial concentration of Pb(II) = 100 mg L−1, pH = 4.0, temperature = 303.15 K, ion-imprinted polymer = 10 mg. | |
3.2.3 Effect of pH value on the adsorption capacity of Pb(II)-IIP. pH value is one of an important factors affecting the amount of adsorption. pH value between 2 and 6 were chosen to explore the effect of pH value on the adsorption capacity in the present work. The effect of different pH values on the adsorption capacity of Pb(II)-IIP was presented in Fig. 6. It showed that pH value strongly affects the adsorption amount of Pb(II)-IIP. When pH value increased from 2 to 4, the adsorption amount increased sharply from 39.8 mg g−1 to 97.5 mg g−1. However, when pH value is increased, the adsorption rate is only reduced by 12.5%. Therefore, the highest adsorption amount was obtained when pH value was 4. Thus, a pH of 4.0 was chosen for further experiments.
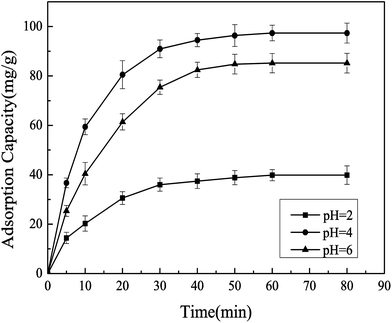 |
| | Fig. 6 Effect of pH values on the adsorption of Pb(II). Conditions: initial concentration of Pb(II) = 100 mg L−1, temperature = 303.15 K, ion-imprinted adsorbent = 10 mg. | |
3.2.4 Effect of temperature on the adsorption capacity. Temperature is another important factor to investigate the effect of temperature on the adsorption capacity. As shown in Fig. 7, the adsorption capacity for Pb(II)-IIP increases obviously from 61.9 mg g−1 to 97.5 mg g−1 when the temperature increases from 293.15 K to 303.15 K, indicating that the temperature has a significant influence on the adsorption capacity. Thus, 303.15 K was chosen as the optimum temperature.
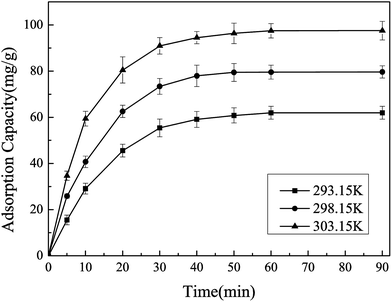 |
| | Fig. 7 Effect of temperatures on the adsorption of Pb(II). Conditions: initial concentration of Pb(II) = 100 mg L−1, pH = 4.0, ion-imprinted adsorbent = 10 mg. | |
3.3 Adsorption kinetics
Two different kinetic models were used to study the adsorption kinetics. They are pseudo-first-order and pseudo-second-order kinetic models.
Based upon the adsorption process controlled by diffusion step, the pseudo-first-order equation was used to describe adsorption behavior of solid–liquid system.18 The equation of pseudo first-order-model can be expressed by eqn (6).
| |
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t Qe − k1t
| (6) |
where
Qe (mg g
−1) and
Qt (mg g
−1) are the adsorption amounts at equilibrium and at time
t (min) respectively.
k1 (min
−1) is pseudo-first-order rate constant of adsorption which is calculated from the slope of the plot obtained by plotting ln(
Qe −
Qt)
versus t, whereas
Qe values were obtained from the intercept of the plot. Corresponding linear regression correlation coefficient
R12 and the rate constant
k1 were listed in
Table 1. The
R12 values are relatively small, while the value of equilibrium adsorption was overestimated, suggesting that the adsorption of lead ions onto Pb(
II)-IIP does not follow the first-order reaction. Despite the fact that pseudo-first-order kinetics model has widely been applied in adsorption processes, the value of adsorption equilibrium exhibits dynamic changes in the actual process. Therefore, the pseudo-first order model cannot accurately estimate the equilibrium adsorption, and can not to satisfactorily describe the adsorption process.
Table 1 Adsorption kinetics parameters of Pb(II) on IIP adsorbent at three different temperatures
| Temperature (K) |
Qe,exp (mg g−1) |
Pseudo-first-order model |
Pseudo-second-order model |
Intraparticle diffusion model |
| R12 |
k1 (min−1) |
Qe,cal (mg g−1) |
R22 |
k2 (g mg−1 min−1) |
Qe,cal (mg g−1) |
Kid (mg g−1 min0.5) |
| 293.15 |
61.96 |
0.9044 |
0.0928 |
81.98 |
0.9725 |
1.22 × 10−3 |
69.93 |
8.323 |
| 298.15 |
79.57 |
0.8782 |
0.0687 |
51.07 |
0.9845 |
1.40 × 10−3 |
87.72 |
7.645 |
| 303.15 |
97.47 |
0.9225 |
0.0640 |
51.15 |
0.9911 |
1.51 × 10−3 |
104.17 |
6.557 |
Because the pseudo-first order model cannot accurately estimate the equilibrium adsorption, the pseudo-second order model applied to analyze the adsorption kinetics can be represented by eqn (7) to describe the adsorption process.
| |
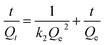 | (7) |
where
k2 (g g
−1 min
−1) presents the rate constant of pseudo-second order adsorption, while all other terms carry the same meanings as previously defined. The rate constant
k2, the value of the equilibrium adsorption
Qe and the correlation coefficient
R22 were calculated from the linear graphs obtained from the plot of
t/
Qt versus t. Results have been summarized in
Table 1.
Linear correlation coefficients and the theoretical value of the equilibrium adsorption were used to choose the best-fit model. As mentioned in Table 1, R2 values for the pseudo-first order kinetic model are 0.8782–0.9225, while the corresponding R2 values for the pseudo-second order kinetic model range from 0.9725–0.9911. In addition, the amounts of equilibrium adsorption increase as the temperature increases. The calculated theoretical adsorption capacity for the pseudo-second order kinetic model is close to the actual adsorption capacity obtained under the same conditions. However, based upon the pseudo-first order kinetic model in Fig. 8, the actual value of the equilibrium adsorption differs from the theoretical value of the amount of adsorption. In short, it was found that pseudo-second order kinetic model provides better correlation for the adsorption of lead ions onto Pb(II)-IIP than the pseudo-first order kinetic model. Therefore, it can be deemed certainly that the adsorption process involves a chemical reaction (Fig. 8).
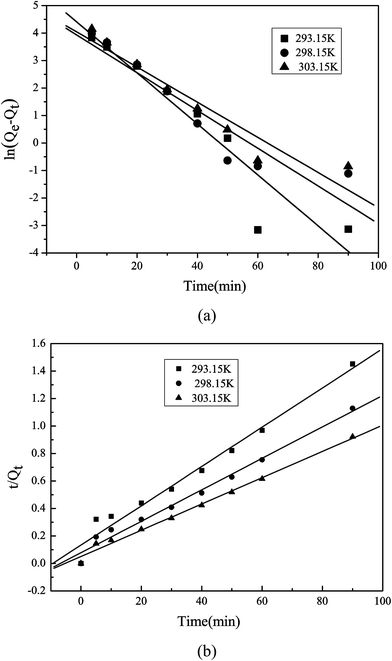 |
| | Fig. 8 Kinetics of the adsorption process for IIP. (a) Pseudo-first order kinetic model (b) pseudo-second order kinetic model. | |
Because the pseudo-first-order and pseudo-second-order models could not analyze the diffusion mechanism accurately, intraparticle diffusion has been selected to investigate the adsorption mechanism. Intraparticle diffusion equation was given as:
where
Qt (mg g
−1) is the amount of adsorption at time
t (min),
Kid (mg g
−1 min
0.5) represents intra-particle diffusion rate constant, which can be calculated from the slope of the plot of
Qt versus t0.5 and the data are shown in
Table 1.
According to the Fig. 9, the changes of intraparticle diffusion model are non-linear in the whole adsorption process, indicating that the adsorption of lead ions in the waste water onto Pb(II)-IIP is not a single way to complete a certain adsorption. The first portion of the curve is attributed to the process of liquid film diffusion, while the second portion is owing to the process of intraparticle diffusion. In addition, the straight line did not go through the origin, suggesting that the intraparticle diffusion is not the only step to control the adsorption rate.
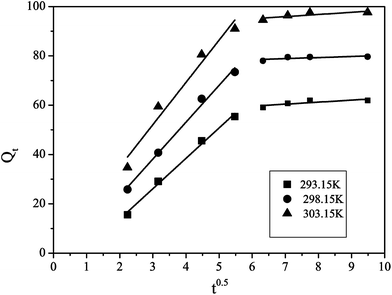 |
| | Fig. 9 Intraparticle diffusion model for Pb-IIP adsorbent. | |
3.4 Adsorption isotherms
Adsorption experiments were carried out to measure the static adsorption at 303.15 K with a series of different initial concentrations. It is important to model the experimental data to obtain adsorption isotherms. In this study, Langmuir isotherm equation and Freundlich equation19 were selected to describe the results of Pb2+ binding on the Pb(II)-IIP. The equations are given as eqn (9) and (10).| |
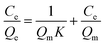 | (9) |
| |
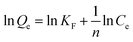 | (10) |
where Qe (mg g−1) and Qm (mg g−1) represent the amount of equilibrium adsorption and the maximum amount of adsorption respectively, while K (L mg−1) is the adsorption equilibrium constant. KF (mg g−1) is the constant of Freundlich model, indicating the adsorption capacity. 1/n represents the adsorption intensity and is called the surface heterogeneity index. A positive but less than unity value of 1/n represents the phenomenon of mono-layer coverage. Otherwise, it is a multi-layer adsorption.
Results of Langmuir and Freundlich isotherm models were shown in Fig. 10. The values of R2 indicate how well the model has fit the experimental data.20 The corresponding linear regression correlation coefficient R2 and other relevant parameters have been listed in Table 2. For Langmuir model, R2 values of Pb(II)-IIP and NIP are 0.9908 and 0.9817 respectively, however R2 values of Freundlich model are 0.8856 and 0.9186 respectively. It could be concluded that R2 value of Langmuir model is higher than that of the Freundlich model. In addition, the calculated value of Qm for the Langmuir model is comparable to the experimental value (Qe). Based on these reasons, it indicated that the adsorption of Pb(II) on Pb(II)-IIP was best described by the Langmuir isotherm model, thus showing that the adsorption of Pb(II) ions on the surface of Pb(II)-IIP is a typical mono-molecular layer adsorption process in which lead ions were adsorbed onto the mono-molecular layer without adsorbent–adsorbate interactions. Furthermore, the Freundlich constant 1/n was smaller than 1, indicating a favorable process.
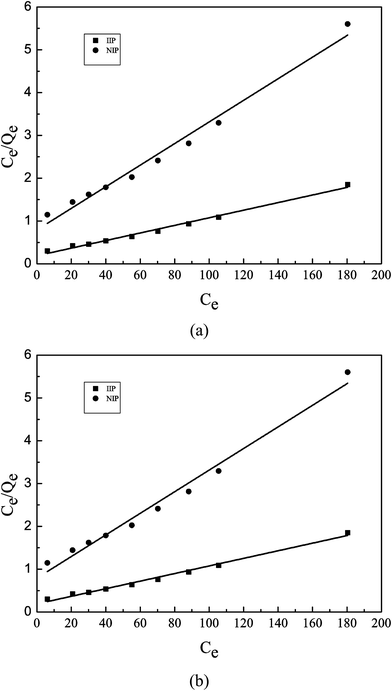 |
| | Fig. 10 Curve fit for the adsorption of Pb(II) onto Pb(II)-IIP using (a) Langmuir model and (b) Freundlich model. | |
Table 2 Adsorption isotherms' parameters for Pb(II) adsorption on Pb(II)-IIP and IIP at 303.15 K
| Adsorbents |
Qe,exp (mg g−1) |
Langmuir model |
Freundlich model |
| R2 |
Qm |
K |
R2 |
1/n |
KF |
| IIP |
97.5 |
0.9908 |
112.35 |
0.0473 |
0.8856 |
0.4761 |
11.10 |
| NIP |
60.02 |
0.9817 |
74.63 |
0.0311 |
0.9186 |
0.5533 |
4.69 |
3.5 Thermodynamic analysis
In order to determine the spontaneous reaction of the adsorption process, the adsorption process of IIP were carried out at different temperatures. Thermodynamic parameters of the adsorption process, changes in standard free energy (ΔG°), changes in standard enthalpy (ΔH°), and changes in standard entropy (ΔS°) were calculated according to eqn (11)–(13).21| |
ΔG° = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc Kc
| (11) |
| |
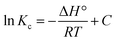 | (12) |
| |
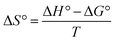 | (13) |
where R is the universal gas constant (8.314 J mol−1 K−1), Kc is an equilibrium constant (Kc = Qe/Ce, mL g−1) and Qe represents the amount of adsorption at equilibrium. ΔH° could be calculated from the slope of the plot obtained by plotting ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc versus 1000/T. The calculated thermodynamic parameters have been presented in Table 3.
Kc versus 1000/T. The calculated thermodynamic parameters have been presented in Table 3.
Table 3 Calculated thermodynamic parameters for Pb(II) adsorption onto IIP adsorbent
| Temperature/K |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc Kc |
ΔG°/kJ mol−1 |
ΔH°/kJ mol−1 |
ΔS°/J mol−1 |
| 293.15 |
6.56 |
−15.99 |
|
190.69 |
| 298.15 |
6.85 |
−16.98 |
39.91 |
190.81 |
| 303.15 |
7.10 |
−17.89 |
|
190.66 |
Table 3 shows that the value of ΔH° is positive at different temperatures, revealing that the adsorption process is endothermic. Therefore, elevating the temperature is helpful to increase the amount of adsorption, which is consistent with the results of kinetic modeling. In addition, the negative values of standard free energy ΔG° depict that the adsorption process could happen spontaneously. Moreover, the values of ΔG° shows a continuous decrease, which showed that temperature of 303.15 K is quite effective for the adsorption process. The changes in standard entropy ΔS° are positive, revealing that the adsorption process results in an increase in entropy of the system. In the process of adsorption, Pb(II) ions adsorbed on the surface of Pb(II)-IIP made the entropy to decrease. However, solvent desorption from the surface of Pb(II)-IIP resulted in an increase in the entropy. It was found that the process of entropy increase is greater than the entropy decrease process, which makes the entropy change a positive quantity.
3.6 Selectivity coefficients
In order to study the selective capacity of Pb(II)-IIP, the binary mixed solutions Pb2+/Cu2+, Pb2+/Cd2+ and Pb2+/Zn2+ were prepared to perform the competitive adsorption experiments under the optimal conditions. According to formulas (3), (4) and (5), the values of the distribution coefficients Kd of each ion and the selectivity coefficients k of Pb(II)-IIP for Pb2+ were listed in Table 4. From Table 4, the selectivity coefficients of Pb(II)-IIP for Pb2+ with respect to Cu2+, Cd2+ and Zn2+ were found to be 4.28, 2.34, and 1.84 respectively. The selectivity coefficients of Pb(II)-IIP for Pb2+ relative to Cu2+, Cd2+ and Zn2+ were found to be 0.61, 0.66 and 0.49 respectively. The relative selectivity coefficients of IIP for Pb2+/Cu2+, Pb2+/Cd2+ and Pb2+/Zn2+ were found to be 7.02, 3.55 and 3.77 respectively. These values suggested that Pb(II)-IIP can selectively adsorb Pb2+ when these ions coexist in the system.
Table 4 Selectivity parameters of IIP adsorbent for Pb2+
| Metals |
Adsorbent |
kd(Pb) (mL g−1) |
kd(X) |
k |
k′ |
| Pb2+/Cu2+ |
IIP |
672.76 |
157.16 |
4.28 |
6.92 |
| NIP |
429.25 |
694.09 |
0.62 |
| Pb2+/Cd2+ |
IIP |
1295.59 |
554.53 |
2.34 |
3.55 |
| NIP |
704.35 |
1058.47 |
0.66 |
| Pb2+/Zn2+ |
IIP |
672.78 |
365.37 |
1.84 |
3.83 |
| NIP |
205.79 |
429.257 |
0.48 |
3.7 Desorption and regeneration
Regeneration and recycling of Pb(II)-IIP is likely to be an important factor in improving the economic efficiency of wastewater treatment, so the adsorption–desorption cycles were repeated five times in this study and shown in Fig. 11. And 6 mol L−1 HCl was effective for removing the adsorbed Pb2+ onto the adsorbent and the elution time was 2 h until Pb2+ was washed out thoroughly. After five recycles, the adsorption capacity of Pb(II)-IIP to Pb2+ is about 7.79% loss in the solution. It indicated that the Pb(II)-IIP could be reused at least five times without significant loss of the adsorption capacities.
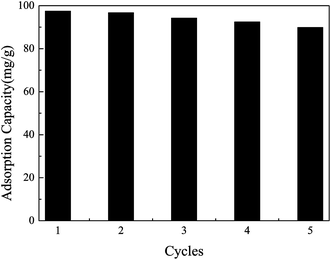 |
| | Fig. 11 Reproducibility of Pb(II)-IIP to Pb2+. | |
4. Conclusions
In this study, a novel Pb(II)-imprinted polymer was synthesized by surface imprinting technique using Pb(II) ion as a template, γ-ureidopropyltrimethoxysilane as the functional monomer, 2,2-azobisisobutyronitrile (AIBN) as initiator and ethyleneglycol dimethacrylate (EGDMA) as cross-linking agent. Pb(II)-IIP was studied for the removal of Pb2+ from aqueous solution. Pb(II)-IIP was thoroughly characterized by FTIR and SEM for the bonding and morphology characteristics respectively. The factors affecting Pb(II)-IIP were investigated. pH of 4, temperature of 303.15 K and time of 60 min were the most optimum for the adsorption of Pb2+. Due to the better correlation with the experimental data, the pseudo-second order kinetic model could accurately describe the adsorption behavior of Pb(II) on Pb(II)-IIP. The kinetics study revealed that the adsorption of Pb(II) on Pb(II)-IIP is a typical mono-molecular layer adsorption. The thermodynamic study indicated that the adsorption process is endothermic in nature and could occur spontaneously. Furthermore, the prepared Pb(II)-IIP was applied to selectively remove Pb2+ from heavy metal wastewater containing foreign coexisting ions. Pb(II)-IIP shows a series of characteristics such as fast adsorption of Pb(II), high adsorption capacity, high recognition selectivity and good reproducibility. It was concluded that Pb(II)-IIP could effectively and satisfactorily remove Pb2+ from heavy metal wastewater.
Acknowledgements
National Natural Science Foundation of China (Project No. 21276174) and Natural Science Foundation of Shanxi province (Project No. 2013011040-1) provided help and support for this work.
References
- B. J. Liu, X. Lv, X. H. Meng, G. L. Yu and D. F. Wang, Chem. Eng. J., 2013, 220, 412 CrossRef CAS.
- P. E. Hande, A. B. Samui and P. S. Kulkarni, Environ. Sci. Pollut. Res., 2015, 22, 7375 CrossRef CAS PubMed.
- L. L. Wang, Y. S. Yan, Y. H. Den, C. X. Li and W. Z. Xu, Chin. J. Anal. Chem., 2009, 37, 537 CAS.
- W. Z. Xu, W. M. Yang and W. Zhou, Adsorpt. Sci. Technol., 2010, 28, 509 CrossRef CAS.
- H. Li, Y. Z. Li, Z. P. Li, X. P. Peng, Y. N. Li, G. Li, X. Z. Tan and G. X. Chen, Appl. Surf. Sci., 2012, 258, 4314 CrossRef CAS.
- H. X. Dong, M. X. Zheng, Y. L. Ou, C. H. Zhang, L. J. Liu, J. Q. Li and X. C. Yang, J. Chromatogr. A, 2015, 1376, 172 CrossRef CAS PubMed.
- T. P. Rao, R. Kala and S. Daniel, Anal. Chim. Acta, 2001, 578, 105 CrossRef PubMed.
- J. Y. Pan, S. Wang and R. F. Zhang, Int. J. Environ. Anal. Chem., 2006, 86, 855 CrossRef CAS.
- Y. Liu, Z. Z. Liu, J. Gao, J. D. Dai, J. Han, Y. Wang, J. M. Xie and Y. S. Yan, J. Hazard. Mater., 2011, 186, 197 CrossRef CAS PubMed.
- R. X. Wang, X. H. Shi, H. J. Wang and C. P. Lei, J. Chem. Eng. Data, 2015, 60, 1454 CrossRef CAS.
- Y. Liu, Z. Z. Liu, Y. Wang, J. D. Dai, J. Gao, J. M. Xie and Y. S. Yan, Microchim. Acta, 2011, 172, 309 CrossRef CAS.
- S. Ghoohestani and H. Faghihian, Desalin. Water Treat., 2016, 57, 4049 CrossRef CAS.
- W. Jiang, H. J. Su, H. Y. Huo and T. W. Tan, Appl. Biochem. Biotechnol., 2010, 160, 467 CrossRef CAS PubMed.
- L. Y. Tong, J. D. Chen, Y. Y. Yuan, Z. H. Cui, M. F. Ran, Q. Zhang, J. Bu, F. Q. Yang, X. H. Su and H. Xu, J. Appl. Polym. Sci., 2015, 132, 1 Search PubMed.
- L. M. Wang, M. H. Zhou, Z. J. Jing and A. F. Zhou, Microchim. Acta, 2009, 165, 367 CrossRef CAS.
- H. T. Fan, X. T. Sun and W. X. Li, J. Sol-Gel Sci. Technol., 2014, 72, 144 CrossRef CAS.
- B. Tabakli, A. A. Topçu, S. Döker and L. Uzun, Ind. Eng. Chem. Res., 2015, 54, 1816 CrossRef CAS.
- J. H. Chen, H. Lin, Z. H. Luo, Y. S. He and G. P. Li, Desalination, 2011, 277, 265 CrossRef CAS.
- J. H. Chen, H. T. Xing, H. X. Guo, G. P. Li, W. Weng and S. R. Hu, J. Hazard. Mater., 2015, 248–249, 285 Search PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 361 CrossRef.
- W. M. Yang, L. K. Liu, Z. P. Zhou, Y. Gao and W. Z. Xu, Res. Chem. Intermed., 2015, 41, 2619 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.  *,
Yanhong Lu and
Luwei Li
*,
Yanhong Lu and
Luwei Li
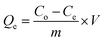
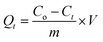
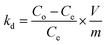
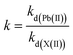
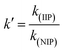
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t
Qe − k1t
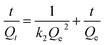

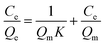
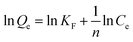

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc
Kc
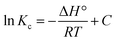
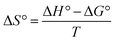
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc versus 1000/T. The calculated thermodynamic parameters have been presented in Table 3.
Kc versus 1000/T. The calculated thermodynamic parameters have been presented in Table 3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc
Kc








