Application of biological-based nano and nano magnetic catalysts in the preparation of arylbispyranylmethanes†
Abstract
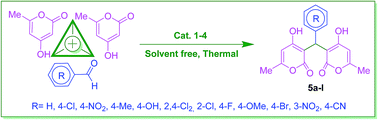
Herein, the utilization of 2-carbamoylhydrazine-1-sulfonic acid, carbamoylsulfamic acid and their related nano magnetic core–shell catalysts were described as biological-based nano catalysts with a urea moiety in the synthesis of arylbispyranylmethane derivatives under mild and eco-friendly reaction conditions. A good range of aromatic aldehydes were treated with 4-hydroxy-6-methyl-2-pyrone to give arylbispyranylmethane derivatives through a tandem Knoevenagel condensation and Michael addition procedure in relatively short reaction times with high yields. The presented protocols have merits like an eco-friendly nature, high efficiency, simple operational procedures and benign reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...