Dielectric and ferroelectric behavior of an incipient ferroelectric Sr(1−3x/2)CexTiO3 novel solid solution†
Abstract
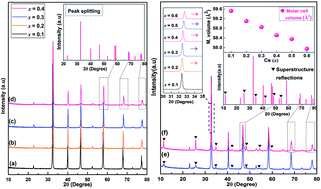
Phase formation, chemical structure, microwave (MW) dielectric properties, and relaxor-to-ferroelectric phase transition behavior of a novel solid solution with Ce-doped, A-site SrTiO3 (Sr(1−3x/2)CexTiO3) ceramic sintered in nitrogen have been investigated. X-ray diffraction (XRD) showed that the samples with x ≤ 0.3 appeared cubic but exhibited splitting and superstructure reflections consistent with tetragonal (0.4 ≤ x ≤ 0.45) and orthorhombic (0.5 ≤ x ≤ 0.6) structures. Chemical structure analysis revealed that the addition of small amounts of ceria (x ≤ 0.3) promoted Ti3+ cation and oxygen vacancy 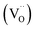 . By contrast, the addition of large amounts of ceria (x ≥ 0.4) inhibited this process. Sr(1−3x/2)CexTiO3 ceramics displayed frequency-dependent relaxor dielectric anomalies and frequency-independent normal ferroelectric behaviors. The relaxor/dielectric properties were strongly dependent on structure-chemical factors. The value of εr decreased several times when Sr was subsequently substituted with Ce, and induced a relaxor-to-ferroelectric phase transition. A good combination of the following microwave dielectric properties was obtained for the Sr0.1Ce0.6TiO3 orthorhombic solid solution at an optimum temperature of 1300 °C for 3 h: εr = 50, Q × f = 11 311 GHz, and τf = +3 ppm per °C.
. By contrast, the addition of large amounts of ceria (x ≥ 0.4) inhibited this process. Sr(1−3x/2)CexTiO3 ceramics displayed frequency-dependent relaxor dielectric anomalies and frequency-independent normal ferroelectric behaviors. The relaxor/dielectric properties were strongly dependent on structure-chemical factors. The value of εr decreased several times when Sr was subsequently substituted with Ce, and induced a relaxor-to-ferroelectric phase transition. A good combination of the following microwave dielectric properties was obtained for the Sr0.1Ce0.6TiO3 orthorhombic solid solution at an optimum temperature of 1300 °C for 3 h: εr = 50, Q × f = 11 311 GHz, and τf = +3 ppm per °C.

 Please wait while we load your content...
Please wait while we load your content...