AIE-active conjugated polymer nanoparticles with red-emission for in vitro and in vivo imaging†
Dongliang Yang‡
a,
Shuwei Zhang‡b,
Yanling Hua,
Jia Chena,
Biqing Baoa,
Lihui Yuwena,
Lixing Weng*c,
Yixiang Cheng*b and
Lianhui Wang*a
aKey Laboratory for Organic Electronics & Information Displays (KLOEID), Institute of Advanced Materials (IAM), Nanjing University of Posts & Telecommunications, 9 Wenyuan Road, Nanjing 210046, China. E-mail: iamlhwang@njupt.edu.cn
bKey Lab of Mesoscopic Chemistry of MOE, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, China. E-mail: yxcheng@nju.edu.cn
cCollege of Geography and Biological Information, Nanjing University of Posts and Telecommunications, Nanjing 210046, China. E-mail: lxweng@njupt.edu.cn
First published on 30th November 2016
Abstract
A novel red-emission conjugated polymer (PBPTPE) with aggregation-induced emission (AIE) characteristics was developed from boron dipyrromethene (BODIPY) derivatives and 1,2-bis(4-ethynylphenyl)-1,2-diphenylethene via palladium-catalyzed Sonogashira coupling reaction. The resulting polymer (PBPTPE) was weakly fluorescent in dichloromethane solution, but showed bright fluorescent emission when aggregated in dichloromethane/hexane mixtures or fabricated into conjugated polymer nanoparticles (PBPTPE NPs). The nanoparticles from PBPTPE were prepared through solvent-exchange method. PBPTPE NPs possess excellent photostability, including superior photobleaching resistance, and good stability in a wide pH range. Besides, in vitro cytotoxicity and hemolysis assay indicated that PBPTPE NPs had favorable biocompatibility. Furthermore, biological evaluation and bioimaging were performed using developmental stage zebrafish embryos. From the survival and hatching rate, oxidative stress and immune-related parameters, no significant adverse effect was observed. The microangiography in zebrafish, further shows that PBPTPE NPs can be used as a bioprobe for future in vivo applications.
Introduction
Fluorescence imaging is a powerful tool in life science research and clinical diagnostics.1–3 Among various luminescent agents, fluorescent nanoparticles have attracted considerable attention due to high brightness, robust photostability and facile surface functionalization as compared with conventional organic dyes.3–5 Optical nanoparticles can be classified into two categories: one is prepared from inorganic elements and the other is fabricated from organic molecules.4 Most of the inorganic nanomaterials such as semiconductor quantum dots, carbon dots, lanthanide-doped and silicon nanoparticles with excellent optical properties (e.g. good photostability, size-tunable emission) are efficacious for biomedical imaging.6–9 However, the inherent limitations of inorganic nanoparticles including potential toxicity, non-biodegradability and accumulated in organisms hampers the clinical applications.10–15 In contrast, fluorescent organic nanoparticles as a new generation of excellent potential bioprobes are fabricated using less toxic organic emitters as the fluorescent core and biocompatible polymers as the encapsulation matrix under mild conditions, which make them attractive candidates for biomedical applications.16–19Motivated by the attractive potentials of fluorescent organic nanoparticles, numerous fluorescent organic nanoparticles based on fluorescent conjugated polymers, AIE dyes and small organic dyes have been extensively investigated in recent years.17,20–22 Conjugated polymer nanoparticles, a kind of highly versatile nanoscale materials, have been widely applied in optoelectronics, photonics, fluorescence imaging, biosensing and biomedicine owning to high brightness, excellent photostability, low cytotoxicity, and versatile surface modification.16 However, the flat aromatic rings structure of fluorophores tends to π–π stacking interaction in aggregation or solid states, which leads to decreased fluorescence emission or poor photostability of the conjugated polymer nanoparticles and limits their potential application for bioimaging.23–25 This phenomenon is more severe in red/near-infrared (R/NIR) emitters.26 As well known, R/NIR probes play a crucial role in biological applications due to their deep penetration, minimum ambient interference and high spatiotemporal resolution.21,27
Currently, many luminogens with AIE characteristics that is opposite to the “aggregation-caused quenching” effect (ACQ) have been developed and used as powerful materials for biomedical applications due to the propeller shape of the molecule hinders π–π stacking and overcomes the ACQ effect. Compared to fluorescent probes based on small molecules which suffer from significant fluorescence quenching in high concentration, AIE active polymer nanoparticles show bright fluorescence emission in aggregated state, high water dispersibility and excellent biocompatibility, making them promising for biomedical applications.16–22,25 Therefore, the approach that introducing AIE luminogens provides a novel platform for developing ultrabright and low cytotoxicity conjugated polymer nanoparticles. In recent years, many R/NIR fluorescent molecules with AIE characteristics were synthesized and fabricated high-performance fluorescent organic nanoparticles with excellent potential for practical applications in live cell and animal imaging.22,27–29 Zebrafish has several noteworthy features over other vertebrate model systems in vivo study.30,31 For example, zebrafish is transparent during early development, which makes them can be used in optical imaging. Further, zebrafish can be easily maintained and generated rapidly at low cost, which can serve as an ideal animal model for screening biocompatibility of nanoparticle probes.30,31
In this work, a novel red emission conjugated polymer (PBPTPE) with AIE characteristics was synthesized from monomers BODIPY derivative (M1) and 1,2-bis(4-ethynylphenyl)-1,2-diphenylethene (M2) via palladium-catalyzed Sonogashira coupling reaction (Scheme 1). The resulting was used to further prepare nanoparticles by the coreprecipitation method with biocompatible 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000). The prepared nanoparticles possess excellent photostability, favorable biocompatibility and can be used for zebrafish imaging.
Experimental section
Materials and measurements
All solvents and reagents were commercially available and analytical reagent grade. Tetrahydrofuran (THF) was distilled from sodium in the presence of benzophenone. NMR spectra were obtained from Bruker Avance 300 spectrometer with 300 MHz for 1H NMR and 75 MHz for 13C NMR and reported as parts per million (ppm) from the internal standard TMS. Mass spectrometry was performed on a SHIMADZU LCMS-2020 Instrument. Fluorescence spectra were obtained from an RF-5301PC spectrometer. Thermogravimetric analyses (TGA) were obtained from a PerkinElmer Pyris-1 instrument under N2 atmosphere. Molecular weight was determined by gel permeation chromatography (GPC) with a Waters 244 HPLC pump, and THF was used as solvent relative to polystyrene standards. M1 and M2 were prepared according to reported methods.32–35Synthesis of polymer PBPTPE
M1 (100.0 mg, 0.11 mmol), M2 (40.0 mg, 0.11 mmol), Pd(PPh3)4 (5% mmol) and CuI (10% mmol) were dissolved in anhydrous THF (10 mL) and Et3N (2 mL). The reaction was stirred at 60 °C for 72 h. After cooling to room temperature, the reaction mixture was filtrated through a short silica gel column. Then the polymer was precipitated in methanol, filtrated and washed with methanol several times. Further purification could be conducted by pouring dense dichloromethane solution into methanol again. The polymer was dried in vacuum to afford PBPTPE as a deep red solid (80.2 mg, 68.9%). GPC: Mw = 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 695, Mn = 13
695, Mn = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 535, PDI = 2.12. 1H NMR (300 MHz, CDCl3): δ 7.89–7.80 (m, 4H), 7.39–7.32 (m, 2H), 7.25–6.90 (m, 28H), 4.09–4.04 (m, 2H), 1.87–1.79 (m, 8H), 1.62–1.28 (m, 16H), 0.93–0.91 (m, 3H).
535, PDI = 2.12. 1H NMR (300 MHz, CDCl3): δ 7.89–7.80 (m, 4H), 7.39–7.32 (m, 2H), 7.25–6.90 (m, 28H), 4.09–4.04 (m, 2H), 1.87–1.79 (m, 8H), 1.62–1.28 (m, 16H), 0.93–0.91 (m, 3H).
Preparation and characterization of PBPTPE NPs
1 mL THF containing PBPTPE (1 mg) and DSPE-PEG2000 (2 mg) was quickly dropped into 10 mL water under robust ultrasonication. The THF in the solution was removed by vigorously stirring in dark environment at room temperature. The resultant suspension was filtered using a 0.22 μm filter and store at 4 °C. The critical micelle concentration of DSPE-PEG2000 was determined to be 8 μM by monitoring micelle turbidity at 240 nm as previous report,36 which was lower than the final concentration of DSPE-PEG2000 (250 μM). Morphology study was carried out with a JEOL 2010 transmission electron microscope at an accelerating voltage of 100 kV. The hydrodynamic size was measured using dynamic light scattering (DLS) with a particle size analyzer (BI-200SM, Brookhaven instruments Corp, Holtsville, NY).Cell culture, in vitro cytotoxicity and imaging studies
Hela cells were purchased from KeyGEN Biotec (Nanjing, China) and cultured in DMEM supplemented with 10% FBS at 37 °C in a humidified incubator with 5% CO2. For live cell fluorescence imaging, the cells were seeded in a 35 mm confocal dish overnight. Next, the cells were incubated with PBPTPE NPs (1 μg mL−1) overnight, then stained with nuclei-specific dye Hoechst 33342 for 5 minutes. Finally, fluorescence images were recorded using confocal microscope under excitation at 405 and 559 nm, and the collected fluorescence channels were 425–525 nm and 580–650 nm, respectively.Cytotoxicity of the PBPTPE NPs against Hela cells were measured by methylthiazolyldiphenyltetrazolium bromide (MTT) assay. 100 μL complete medium with 5 × 104 Hela cells was seeded in 96-well plates. After incubated 24 h, the culture medium was discarded and the cells were treated with DMEM medium containing different concentrations PBPTPE NPs (1, 1.25, 2.5, 5 μg mL−1) for 24 or 48 h. Next, the medium was removed and 100 μL fresh DMEM medium containing MTT (0.5 mg mL−1) was added to each well, followed by incubation for 4 h at 37 °C. Then MTT solution was removed and 150 μL DMSO was added to each well to dissolve the precipitates formed. Then the absorbance of each well at 570 nm was measured using a Microplate Spectrophotometer (PowerWave XS2, BIOTEK, US). Cell viability was evaluated by the ratio of the absorbance of the cells treated with lipid-P2 NPs PBPTPE NPs to that of the cells incubated with culture medium only.37
Fish husbandry
Zebrafish (Danio rerio) were raised in a recirculating tank filled with a buffered solution (60 mg L−1 instant ocean salts) at 27 °C with 14 h/10 h light/dark cycle. Adult zebrafish were fed brine shrimp three times a day. Males and females (ratio = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were placed together in small breeding tanks the night before eggs were required. The embryos were collected, pooled and rinsed at least three times with E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4, pH 7.4). All animal experiments were conducted in accordance with the guidelines of the National Institutes of Health on animal care and approved by the Animal Ethics Committees of Nanjing University.
1) were placed together in small breeding tanks the night before eggs were required. The embryos were collected, pooled and rinsed at least three times with E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4, pH 7.4). All animal experiments were conducted in accordance with the guidelines of the National Institutes of Health on animal care and approved by the Animal Ethics Committees of Nanjing University.
Hemolysis test and in vivo imaging
Red blood cells were collected from the adult zebrafish as described.38 Twenty adult zebrafish were anesthetized with 0.2% tricaine in E3 medium. Then using the scalpel to make an incision between the anal and caudal fin in the anesthetized fish; blood coming out of the wound was collected with the Eppendorf pipettor and pooled in an EDTA-coated microtube. Red blood cells were sedimented by centrifugation at 2000 rpm for 5 minutes and washed thrice with sterile 0.9% NaCl saline solution. Further, the red blood cells were resuspended in 450 μL 0.9% NaCl solution. For hemolysis assay, 100 μL of red blood cells were mixed with 900 μL 0.9% NaCl solution containing different concentrations PBPTPE NPs and kept 37 °C for 2 h. Besides, water and 0.9% NaCl were used as the positive and negative control. For intravital imaging, zebrafish larvae were anesthetized with 0.05% tricaine and arrayed in the trenches of the microinjection chamber. Zebrafish larvae were individually injected 100 nL PBPTPE NPs (20 μg mL−1) through the ventricle and transferred into 24-well plates until fluorescence imaging.39Nanoparticle exposure
Healthy embryos were placed in 96-well flat-bottom tissue culture plates (1 embryo in 200 μL solution each well, 25 embryos per group). The nanoparticle stock solution (100 μg mL−1) was diluted with E3 medium. Then embryos were randomly divided into four groups. The control group was cultured in E3 medium, whereas the other groups were exposed to (1, 10, 20 μg mL−1) PBPTPE NPs. The microscopic observation was performed in the well under a microscope attached to a CCD camera every 12 hours. The surviving and hatching rate were recorded at each time point.Analysis of oxidative stress and immune-related parameters
After exposure 84 hours post fertilization (hpf), zebrafish embryos were washed with E3 medium and were homogenized using a homogenizer in phosphate buffered saline (PBS). Then the sample was performed according to the kit manufacturer's instructions. Malondialdehyde (MDA) content, catalase (CAT) activity, total antioxidant capacity (T-AOC), nitric oxide (NO) level, lysozyme (LZM) and alkaline phosphatase (AKP) activity were determined using commercially kits that order from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). The content of MDA was detected by the thiobarbituric acid reactive substances method.40 CAT activity was determined by the decomposition of hydrogen peroxide method.41 The total-antioxidant capacity was detected using the ferric reducing antioxidant power (FRAP) assay.42 NO level was detected by the Griess reaction.43 LZM was detected using the classical turbidity assay.44 AKP activity was measured by a colorimetric method that developed by Boehringer.45Result and discussion
Synthesis and characterization
As shown in Scheme S1,† PBPTPE was synthesized from M1 and M2 via palladium-catalyzed Sonogashira coupling reaction.32 The NMR spectra of M1 and M2 were showed in Fig. S1–S3 in ESI.† The chemical structure of PBPTPE was characterized by 1H NMR and the weight average molecular weight (Mw) of the polymer was 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 695 (PDI = 2.12). The polymer can dissolve in common organic solvents, such as THF, dichloromethane, acetonitrile.
695 (PDI = 2.12). The polymer can dissolve in common organic solvents, such as THF, dichloromethane, acetonitrile.
The photophysical property of PBPTPE in a series of dichloromethane–hexane mixed solvents was measured using UV-vis absorption and photoluminescence (PL) spectroscopy. As shown in Fig. 1c, PBPTPE in dichloromethane solution has two strong absorption peaks at 347 nm and 537 nm, and the absorbance intensities show a little change as the ratio of bad solution increasing (Fig. S4†). While the PL peak intensities at 610 nm show increase dramatically and reach its maximum at 90% hexane content, which is 6-fold higher than the pure dichloromethane solution (Fig. S5†). These results indicate the polymer possessing obvious AIE features.46 And the polymer displays a large Stokes shift of ∼79 nm, which is compared to regular BODIPY dyes (7–15 nm).47
Preparation and characterization of PBPTPE NPs
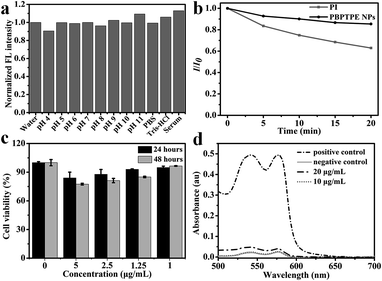
PBPTPE NPs were formed by the nanoprecipitation method as described previously.25 To investigate the particles' morphology and size, the PBPTPE NPs were characterized by TEM and DLS. Fig. 1a shows that the particles are spherical under the preparation condition, and the DLS result indicates that the average hydrodynamic diameter of PBPTPE NPs is about 35 nm. The UV-vis spectra of PBPTPE NPs in aqueous solution and PBPTPE in dichloromethane solution were showed in Fig. 1c. The absorbance peak of PBPTPE NPs aqueous solution at 544 nm exhibits a weak red-shift of 9 nm compare to PBPTPE in dichloromethane solution. PL spectra reveal an emission peak of the PBPTPE NPs red-shift from 610 to 625 nm (Fig. 1d). These phenomena may be ascribed to the strong intermolecular π–π interactions.37 The fluorescent quantum yields of PBPTPE NPs in water was measured to be 0.11, using rhodamine 6G in water as the standard.48As we all know, fluorescent probes with high photostability are important to imaging, because the spatial resolution is depend on the number of photons collected from the probes.4 In general terms, the label microenvironment including protein, pH and ionic strength affects the photochemical stability of the nanoparticles.49 However, label stability is important for detection sensitivity, especially in single molecule fluorescence imaging.49 For evaluating the stability of PBPTPE NPs, PBPTPE NPs were added to different environments such as PBS, Tris–HCl, 10% serum and pH 4 to 11 solutions. PL spectra were recorded and presented in Fig. 2a. Fig. 2a shows that the fluorescence intensities of PBPTPE NPs have no obvious change. The encapsulation matrix DSEG-lipid greatly increases the stability of PBPTPE NPs.50 These results are consistent with previous reports.51 Further, the photostability of PBPTPE NPs was measured and compared to commercially nuclear dye propidium iodide (PI) due to the same excitation wavelength. As shown in Fig. 2b, PBPTPE NPs exhibit an excellent photostability than that of PI. After 20 minutes illumination at 535 nm, 85% of fluorescence of PBPTPE NPs could be maintained, while that of PI decrease to 63%. These results demonstrate that PBPTPE NPs are great potential for bioimaging in vitro and in vivo.
Cytotoxicity and in vitro cell imaging
To examine the utility of PBPTPE NPs in bioimaging application, the cytotoxicity of PBPTPE NPs to Hela cells was examined by MTT assay. After 24 h incubation, the cell viability was greater than 85%, even at a concentration of 5 μg mL−1; while extended the incubate time to 48 h, the cell viability shows a slight reduction, except the concentration at 1 μg mL−1. These results show that PBPTPE NPs have a good biocompatibility. Based on the cell cytotoxicity assessment, PBPTPE NPs have favorable cytocompatibility for bioimaging applications due to the prominent properties of PEG including excellent water soluble, nontoxic and biocompatible properties.20 We used Hela cell as a model for PBPTPE NPs in cell imaging, and incubated the cells with PBPTPE NPs (1 μg mL−1) overnight, then stained with Hoechst 33342. As shown in Fig. 3, PBPTPE NPs were mainly distributed in cytoplasm but not their nuclei. Further, we found PBPTPE NPs were effectively taken up when the Hela cells incubated with 5 μg mL−1 PBPTPE NPs for 2 h due to intense red fluorescence was observed in cells surrounding the cellular nucleus (Fig. S6†). The uptake of PBPTPE NPs maybe though endocytosis, then were released to the cytoplasm from the endosomes and lysosomes.52Hemolysis assay and in vivo imaging
Red blood cell destruction leads to anemia, jaundice, and reticulocytosis.53,54 Before PBPTPE NPs were used for microangiography in zebrafish, we firstly evaluated the nanomaterials biocompatibility with blood components. Fig. 2d shows only 5.6% hemolysis when erythrocytes were exposed to PBPTPE NPs (10 μg mL−1). The lower hemolytic activity of PBPTPE NPs is attributed to the biocompatible encapsulation matrix.20 This result shows that PBPTPE NPs have a good hemocompatibility (Fig. 4a), which provides great advantage for their biomedical applications.51 As well known, the cardiovascular system is responsible for transporting nutrients and moving heat.55 In order to examine the properties of PBPTPE NPs in vivo, PBPTPE NPs were injected into the ventricle of the zebrafish due to the transparent zebrafish embryos.30 Then the nanomaterials were delivered in blood system. The vascular network of zebrafish was seen from Fig. 4b under a fluorescence microscope. This further confirms PBPTPE NPs can be used as a bioprobe for microangiography in zebrafish.Toxicity assessment in zebrafish embryos
PBPTPE NPs show low toxicity against Hela cells and red blood cells. Furthermore, the potential effect of PBPTPE NPs on zebrafish embryo development was evaluated. Survival rate of embryos, are shown in Fig. 5a after PBPTPE NPs treatment from 12 to 134 hpf. No obvious adverse effect was observed on embryonic survival when embryos incubated with PBPTPE NPs (lower than 20 μg mL−1). In the hatching experiment, in the PBPTPE NPs exposure (low than 10 μg mL−1) and control group, all the surviving embryo hatching began at 60 and finished at 120 hpf. PBPTPE NPs at concentration 20 μg mL−1 showed a hatching delay that may be cause by hypoxia and decreased activity of hatching enzyme,13 while extended the incubate time to 132 hpf can rescue this adverse (Fig. 5b). These results indicate that PBPTPE NPs have no obvious adverse effect in zebrafish early embryonic development. To further investigate PBPTPE NPs' distribution, zebrafish embryos were observed after 24 h of exposure under a confocal laser scanning microcopy. As Fig. S7† shown, the PBPTPE NPs adsorbed to the chorion of zebrafish embryos after 24 h of exposure. When the embryos hatched, no fluorescence was observed in larvae under the fluorescent microscope. Furthermore, the oxidative stress-related parameters, including MDA content as an indicator of oxidative damage, CAT activity and T-AOC were investigated.40–42 As Fig. 5c shown, no significant changes happened in the levels of MDA and CAT after nanoparticles treatment; but T-AOC upregulated about 15% when exposed to 10 and 20 μg mL−1 PBPTPE NPs. These results indicate PBPTPE NPs may block the pore canals of the chorion and cause hypoxia owing to PBPTPE NPs adsorbed to the chorion at high concentration. Similar results also are discovered when zebrafish was exposed to graphene oxide and ZnO nanoparticles.14,56 Then the immune-related parameters in zebrafish were measured after the zebrafish exposed to PBPTPE NPs. Nitric oxide (NO), a messenger molecule, plays vital role in innate immune, vascular and nervous systems.57–59 LZM and APK, both hydrolase enzymes, are also involved in immune, antibacterial activities.60–62 As shown in Fig. 5d, NO level, LZM and APK activities in zebrafish larvae were no influence. These results indicate that PBPTPE NPs have no obvious adverse effect in innate immune response systems and anti-oxidant system in zebrafish, further confirm PBPTPE NPs possess favorable biocompatibility in zebrafish.Conclusions
In this study, an AIE-active conjugated polymer was first synthesized from 1,2-bis(4-ethynylphenyl)-1,2-diphenylethene and BODIY-based derivative by palladium-catalyzed Sonogashira coupling reaction. Then conjugated polymer particles (PBPTPE NPs) were fabricated via co-precipitation method from AIE-active conjugated polymer PBPTPE and DSPE-PEG2000. The PBPTPE NPs show excellent photostability and good stability in wide pH range with red emission. Furthermore, in vitro and vivo experimental results indicated that the PBPTPE NPs had good cytocompatibility, hemocompatibility, immunocompatibility and can be used in vivo imaging. These merits make the PBPTPE NPs promising fluorescent probe for bioimaging applications.Acknowledgements
This study was supported by the National Basic Research Program of China (2012CB933301), National Natural Science Foundation of China (81273409, 21204038).References
- R. Weissleder and M. J. Pittet, Nature, 2008, 452, 580–589 CrossRef CAS PubMed.
- M. Koch and V. Ntziachristos, Annu. Rev. Med., 2016, 67, 153–164 CrossRef CAS PubMed.
- K. Pu, N. Chattopadhyay and J. Rao, J. Controlled Release, 2016, 240, 312–322 CrossRef CAS PubMed.
- H.-S. Peng and D. T. Chiu, Chem. Soc. Rev., 2015, 44, 4699–4722 RSC.
- O. S. Wolfbeis, Chem. Soc. Rev., 2015, 44, 4743–4768 RSC.
- Y. He, H. Lu, Y. Su, L. Sai, M. Hu, C. Fan and L. Wang, Biomaterials, 2011, 32, 2133–2140 CrossRef CAS PubMed.
- D. K. Chatterjee, A. J. Rufaihah and Y. Zhang, Biomaterials, 2008, 29, 937–943 CrossRef CAS PubMed.
- Y. He, Y. Zhong, F. Peng, X. Wei, Y. Su, Y. Lu, S. Su, W. Gu, L. Liao and S.-T. Lee, J. Am. Chem. Soc., 2011, 133, 14192–14195 CrossRef CAS PubMed.
- R. Hardman, Environ. Health Perspect., 2006, 114, 165–172 CrossRef PubMed.
- J. Tian, X. Zeng, X. Xie, S. Han, O.-W. Liew, Y.-T. Chen, L. Wang and X. Liu, J. Am. Chem. Soc., 2015, 137, 6550–6558 CrossRef CAS PubMed.
- X. Li, X. Yang, L. Yuwen, W. Yang, L. Weng, Z. Teng and L. Wang, Biomaterials, 2016, 96, 24–32 CrossRef CAS PubMed.
- T. M. Scown, E. M. Santos, B. D. Johnston, B. Gaiser, M. Baalousha, S. Mitov, J. R. Lead, V. Stone, T. F. Fernandes, M. Jepson, R. van Aerle and C. R. Tyler, Toxicol. Sci., 2010, 115, 521–534 CrossRef CAS PubMed.
- W. Bai, Z. Zhang, W. Tian, X. He, Y. Ma, Y. Zhao and Z. Chai, J. Nanopart. Res., 2009, 12, 1645–1654 CrossRef.
- Y. Chen, X. Hu, J. Sun and Q. Zhou, Nanotoxicology, 2016, 10, 42–52 CAS.
- R. Werlin, J. H. Priester, R. E. Mielke, S. Kramer, S. Jackson, P. K. Stoimenov, G. D. Stucky, G. N. Cherr, E. Orias and P. A. Holden, Nat. Nanotechnol., 2011, 6, 65–71 CrossRef CAS PubMed.
- L. Feng, C. Zhu, H. Yuan, L. Liu, F. Lv and S. Wang, Chem. Soc. Rev., 2013, 42, 6620–6633 RSC.
- Z. Zhao, B. Chen, J. Geng, Z. Chang, L. Aparicio-Ixta, H. Nie, C. C. Goh, L. G. Ng, A. Qin, G. Ramos-Ortiz, B. Liu and B. Z. Tang, Part. Part. Syst. Charact., 2014, 31, 481–491 CrossRef CAS.
- X. Zhang, X. Zhang, S. Wang, M. Liu, L. Tao and Y. Wei, Nanoscale, 2013, 5, 147–150 RSC.
- X. Zhang, K. Wang, M. Liu, X. Zhang, L. Tao, Y. Chen and Y. Wei, Nanoscale, 2015, 7, 11486–11508 RSC.
- X. Zhang, X. Zhang, B. Yang, S. Wang, M. Liu, Y. Zhang, L. Tao and Y. Wei, RSC Adv., 2013, 3, 9633–9636 RSC.
- X. Zhang, X. Zhang, B. Yang, J. Hui, M. Liu, Z. Chi, S. Liu, J. Xu and Y. Wei, Polym. Chem., 2014, 5, 318–322 RSC.
- H. Lu, Y. Zheng, X. Zhao, L. Wang, S. Ma, X. Han, B. Xu, W. Tian and H. Gao, Angew. Chem., Int. Ed. Engl., 2016, 55, 155–159 CrossRef CAS PubMed.
- A. Reisch and A. S. Klymchenko, Small, 2016, 12, 1968–1992 CrossRef CAS PubMed.
- D. Yang, F. Li, Z. Luo, B. Bao, Y. Hu, L. Weng, Y. Cheng and L. Wang, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 1686–1693 CrossRef CAS.
- K. Li and B. Liu, Chem. Soc. Rev., 2014, 43, 6570–6597 RSC.
- D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441–2453 CrossRef CAS PubMed.
- C. Dai, D. Yang, W. Zhang, B. Bao, Y. Cheng and L. Wang, Polym. Chem., 2015, 6, 3962–3969 RSC.
- X. Zhang, X. Zhang, B. Yang, Y. Zhang and Y. Wei, Tetrahedron, 2014, 70, 3553–3559 CrossRef CAS.
- Z. Wang, L. Yan, L. Zhang, Y. Chen, H. Li, J. Zhang, Y. Zhang, X. Li, B. Xu, X. Fu, Z. Sun and W. Tian, Polym. Chem., 2014, 5, 7013–7020 RSC.
- S.-K. Ko, X. Chen, J. Yoon and I. Shin, Chem. Soc. Rev., 2011, 40, 2120–2130 RSC.
- K. J. Lee, P. D. Nallathamby, L. M. Browning, C. J. Osgood and X.-H. N. Xu, ACS Nano, 2007, 1, 133–143 CrossRef CAS PubMed.
- S. Zhang, Y. Wang, F. Meng, C. Dai, Y. Cheng and C. Zhu, Chem. Commun., 2015, 51, 9014–9017 RSC.
- C. Tahtaoui, C. Thomas, F. Rohmer, P. Klotz, G. Duportail, Y. Mely, D. Bonnet and M. Hibert, J. Org. Chem., 2007, 72, 269–272 CrossRef CAS PubMed.
- S. Zhang, Y. Sheng, G. Wei, Y. Quan, Y. Cheng and C. Zhu, Polym. Chem., 2015, 6, 2416–2422 RSC.
- W. Z. Yuan, F. Mahtab, Y. Gong, Z.-Q. Yu, P. Lu, Y. Tang, J. W. Y. Lam, C. Zhu and B. Z. Tang, J. Mater. Chem., 2012, 22, 10472–10479 RSC.
- T. Ishida, D. L. Iden and T. M. Allen, FEBS Lett., 1999, 460, 129–133 CrossRef CAS PubMed.
- M. Lan, J. Zhang, X. Zhu, P. Wang, X. Chen, C.-S. Lee and W. Zhang, Nano Res., 2015, 8, 2380–2389 CrossRef CAS.
- J. M. Murtha, W. Qi and E. T. Keller, Comp. Med., 2003, 53, 37–41 CAS.
- S. W. Son, J. H. Kim, S. H. Kim, H. Kim, A. Y. Chung, J. B. Choo, C. H. Oh and H. C. Park, Skin Res. Tech., 2009, 15, 157–160 CrossRef PubMed.
- L. Xu, X. Yang, J. Chen, X. Ge, Q. Qin, H. Zhu, C. Zhang and X. Sun, Drug Des., Dev. Ther., 2016, 10, 2271–2278 CrossRef PubMed.
- M. Alía, S. Ramos, R. Mateos, L. Bravo and L. Goya, J. Biochem. Mol. Toxicol., 2005, 19, 119–128 CrossRef PubMed.
- I. F. F. Benzie and J. J. Strain, Anal. Biochem., 1996, 239, 70–76 CrossRef CAS PubMed.
- I. Guevara, J. Iwanejko, A. Dembińska-Kieć, J. Pankiewicz, A. Wanat, P. Anna, I. Gołbek, S. Bartuś, M. Malczewska-Malec and A. Szczudlik, Clin. Chim. Acta, 1998, 274, 177–188 CrossRef CAS.
- R. Helal and M. F. Melzig, Pharmazie, 2008, 63, 415–419 CAS.
- P. Garnero and P. D. Delmas, J. Clin. Endocrinol. Metab., 1993, 77, 1046–1053 CAS.
- R. Hu, C. F. Gomez-Duran, J. W. Lam, J. L. Belmonte-Vazquez, C. Deng, S. Chen, R. Ye, E. Pena-Cabrera, Y. Zhong, K. S. Wong and B. Z. Tang, Chem. Commun., 2012, 48, 10099–10101 RSC.
- J. F. Araneda, W. E. Piers, B. Heyne, M. Parvez and R. McDonald, Angew. Chem., Int. Ed. Engl., 2011, 50, 12214–12217 CrossRef CAS PubMed.
- D. Magde, R. Wong and P. G. Seybold, Photochem. Photobiol., 2002, 75, 327–334 CrossRef CAS PubMed.
- U. Resch-Genger, M. Grabolle, S. Cavaliere-Jaricot, R. Nitschke and T. Nann, Nat. Methods, 2008, 5, 763–775 CrossRef CAS PubMed.
- H. Hatakeyama, H. Akita and H. Harashima, Adv. Drug Delivery Rev., 2011, 63, 152–160 CrossRef CAS PubMed.
- D. Li, X. Zhao, W. Qin, H. Zhang, Y. Fei, L. Liu, K.-T. Yong, G. Chen, B. Z. Tang and J. Qian, Nano Res., 2016, 9, 1921–1933 CrossRef CAS.
- S. D. Conner and S. L. Schmid, Nature, 2003, 422, 37–44 CrossRef CAS PubMed.
- M. A. Dobrovolskaia, J. D. Clogston, B. W. Neun, J. B. Hall, A. K. Patri and S. E. McNeil, Nano Lett., 2008, 8, 2180–2187 CrossRef CAS PubMed.
- C. H. Packman, Blood Rev., 2008, 22, 17–31 CrossRef CAS PubMed.
- E. G. Lakatta, in Compr Physiol, John Wiley & Sons, Inc., 2010, DOI:10.1002/cphy.cp110117.
- X. Zhao, S. Wang, Y. Wu, H. You and L. Lv, Aquat. Toxicol., 2013, 136–137, 49–59 CrossRef CAS PubMed.
- G. B. Stefano, Y. Goumon, T. V. Bilfinger, I. D. Welters and P. Cadet, Prog. Neurobiol., 2000, 60, 513–530 CrossRef CAS PubMed.
- F. B. Jensen, J. Exp. Biol., 2007, 210, 3387–3394 CrossRef CAS PubMed.
- C. Bogdan, M. Rollinghoff and A. Diefenbach, Immunol. Rev., 2000, 173, 17–26 CAS.
- Z. Wang and S. Zhang, Fish Shellfish Immunol., 2010, 29, 773–777 CrossRef CAS PubMed.
- W. Carrillo, J. A. Gómez-Ruiz, B. Miralles, M. Ramos, D. Barrio and I. Recio, Eur. Food Res. Technol., 2016, 242, 1777–1785 CrossRef CAS.
- W. Dang, W. Zhang and W. G. Du, Sci. Rep., 2015, 5, 10594 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR spectra of compounds, absorbance spectra and fluorescent spectra of PBPTPE, fluorescent microscopy images of the zebrafish embryos exposed to PBPTPE NPs. See DOI: 10.1039/c6ra18678e |
| ‡ These authors contributed equally to this article. |
| This journal is © The Royal Society of Chemistry 2016 |