DOI:
10.1039/C6RA18635A
(Paper)
RSC Adv., 2016,
6, 78505-78513
Formation of lipid membrane-incorporated small π-molecules bearing hydrophilic groups†
Received
22nd July 2016
, Accepted 12th August 2016
First published on 12th August 2016
Abstract
Nineteen poorly water-soluble π-conjugated molecules were evaluated in terms of their ability to be stably incorporated into lipid membranes. The resulting lipid membrane-incorporated π-conjugated guest molecules (LMIGs) were classified into four categories, including (i) those that formed stable LMIGs; (ii) those where some of the guest molecules precipitated; (iii) those that formed small self-aggregates consisting of lipids and/or guest molecules; and (iv) those that leaked some of the guest molecules into the bulk water. Compounds belonging to category (ii) were confirmed by UV-vis absorption analysis. In contrast, compounds belonging to categories (i), (iii) and (iv) were discriminated based on their 1H NMR spectra and the broadening or disappearance of the peaks of the guest molecules and the lipids in LMIGs and large liposomes. Several LMIGs could be converted from one category to another using other lipids. Furthermore, the guest molecules belonging to category (iv) were successfully predicted using the octanol–water partition coefficient, which was calculated by simulation.
1. Introduction
Liposomes have recently attracted considerable interest as suitable materials for the development of drug-delivery systems (DDSs), where therapeutic agents can be encapsulated by a vesicle or a polymeric material (e.g., DNA) can be adsorbed on the outside of a vesicle.1–9 The advantages of liposomes include their lower cost, ease of large-scale manufacture and ability to evade immune and inflammatory responses.10 However, it is not yet known whether poorly water-soluble π-conjugated drug molecules can be incorporated into lipid membranes. There are two main factors involved in determining the stability (or formation) of lipid membrane-incorporated π-conjugated guest molecules (LMIGs), including (i) the affinity of the guest molecule for the alkyl chains of the lipids; and (ii) the self-aggregation of the guest molecules in the lipid membranes. Factor (ii) can affect the long-term stability of LMIG solutions, which can be improved by inhibiting the self-aggregation of the guest molecules.11,12 In contrast, factor (i) determines whether all of the guest molecules can form LMIGs. In the case of there being low affinity between the guest molecules and the alkyl chains of the lipids, the hydrophobic guest molecules can precipitate from solution outside of the lipid membranes by conventional methods. However, we recently succeeded in preparing LMIGs from several hydrophobic guest molecules without the formation of any precipitates from the prerequisite cyclodextrin complexes using the exchange method in lipid membranes.11–26 However, the use of guest compounds bearing a hydrophilic moiety could result in some leakage from the lipid membranes. This type of leakage is a very important factor for the preparation of LMIGs based on guest molecules or drugs and liposomes. Schwarzenbach et al. recently reported a method for determining the liposome-water distribution ratios (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Klipw) of substituted phenols and several other compounds.27–31 Although this value can be useful for evaluating the affinity of a guest molecule for lipid and cell membranes, it still remains difficult to determine whether guest molecules have been successfully incorporated into liposomes at high concentrations. In this paper, we have 1H NMR spectroscopy to determine the leakage percentages of guest molecules from liposomes. The results of this study have shown that LMIGs were not only labilized by the guest molecules towards leaking their guest molecules into water but that they also formed small self-aggregates with lipids. Furthermore, we have investigated the relationship between the equilibrium and the octanol–water partition coefficient (log
Klipw) of substituted phenols and several other compounds.27–31 Although this value can be useful for evaluating the affinity of a guest molecule for lipid and cell membranes, it still remains difficult to determine whether guest molecules have been successfully incorporated into liposomes at high concentrations. In this paper, we have 1H NMR spectroscopy to determine the leakage percentages of guest molecules from liposomes. The results of this study have shown that LMIGs were not only labilized by the guest molecules towards leaking their guest molecules into water but that they also formed small self-aggregates with lipids. Furthermore, we have investigated the relationship between the equilibrium and the octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow) for some model small π-compounds (1–19) and confirmed the threshold values log
Pow) for some model small π-compounds (1–19) and confirmed the threshold values log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow for these systems.28
Pow for these systems.28
2. Experimental
2.1. Experimental materials
trans-Stilbene (1), 4-styrylpyridine (2), 4-phenylpyridine (4), 4,4′-bipyridyl (5), 2,2′-bipyridyl (6) and 2,3-naphthalenedicarboxylic acid (12) were purchased from Wako Pure Chemical Industries Ltd (Tokyo, Japan). Biphenyl (3), 1,4-di(4-pyridyl)benzene (7), 2-naphthol (8), naphthalene-2,7-diol (9), 1,1′-bi-2-naphthol (10), 2-naphthoic acid (11), 6-hydroxy-2-naphthoic acid (13), 2-naphthalenecarbaldehyde (14), naphthalene-1-amine (15), naphthalene-1,8-diamino (16), coumarin (17), 6-methylcoumarin (18) and phthalide (19) were purchased from Tokyo Chemical Industries Co., Ltd. (Tokyo, Japan). 1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) were obtained from NOF Corp. (Tokyo, Japan) and Avanti Polar Lipids, Inc. (Alabaster, AL, USA), respectively.
2.2. Preparation of lipid membrane-incorporated guest molecules (LMIGs)
Solutions of DMPC or DPPC (4.00 × 10−6 mol) and compounds 1–19 in chloroform (0.1 mL) were concentrated on a rotary evaporator at 40 °C. The compositions of the mixtures were as follows: [1–19]/[DMPC] = 5.0, 10.0 and 20.0 mol%. Pure water (1.0 mL) was added and the aqueous mixture was agitated on a vortex mixer for 1 min. To resulting multilamellar vesicles were subjected to eight freeze–thaw cycles and extruded eleven times (LiposoFast-Basic; Avestin Inc., Ottawa, Canada) through two stacked polycarbonate membranes (pore size 50 nm) to afford unilamellar vesicles. The final lipid concentration was 4.0 mM.
2.3. Cryogenic-temperature transmission electron microscopy (Cryo-TEM)
Cryo-TEM samples were prepared using a universal cryofixation and cryopreparation system (Leica EM CPC, Wetzlar, Germany). The isolated chamber was humidified to near saturation prior to the introduction of the sample to avoid the evaporation of water from the sample. Sample droplets (2–3 mL) were placed on a microperforated cryo-TEM grid and absorbed on to a filter paper. This process resulted in the formation of thin liquid films of 10–300 nm in thickness that freely spanned the micropores of a carbon-coated lacelike polymer layer supported by a metal mesh grid. After a minimum hold time of 30 s, the sample grid assembly was rapidly vitrified with liquid ethane at its melting temperature (−163 to −170 °C). A hold time was adopted to relax any deformations in the flow that may have occurred during the blotting process. The vitreous specimen was kept under liquid nitrogen prior to being loaded into a cryogenic sample holder (Gatan 626-DH). Imaging was performed on a JEOL JEM-3100 FEF instrument operating at 300 kV (Tokyo, Japan). The use of a minimal dose system (MDS) was necessitated by the electron radiation sensitivity of the sample being probed. Images were recorded on a Gatan 794 multiscan digital camera (Gatan Inc., Pleasanton, CA, USA) and processed using version 3.8.1 of the DigitalMicrograph software (Gatan Inc., Pleasanton, CA, USA). The optical density gradients of the background, which are normally ramp-shaped, were digitally corrected using a custom-made subroutine that is compatible with DigitalMicrograph.
3. Results and discussion
3.1. Formation of LMIGs by the premixing method
Lipid membrane-incorporated compounds 1–19 (LMI1–19) consisting of dimyristoylphosphatidylcholine (DMPC) were prepared according to a previously reported premixing method (Scheme S1†).10,32 Briefly, the LMIGs were prepared by dissolving the lipids and the guest molecules in a suitable organic solvent, followed by concentration of the resulting mixture to give a residue, which was extracted with water. Most of the benzene derivatives were evaporated during the concentration step together with the organic solvents. For this reason, we used naphthalene, biphenyl and coumarin derivatives or their analogues as guest molecules. LMI1 has been reported previously.33,34
3.2. Aqueous solubilization of guest molecules
3.2.1 The effect of adding pyridyl groups. 4-Styrylpyridine (2), 4-phenylpyridine (4), 4,4′-bipyridine (5), 2,2′-bipyridine (6) and 1,4-di(4-pyridyl)benzene (7) were used as guest molecules to investigate the impact of adding a hydrophilic pyridyl moiety. The solubilities of compounds 2 and 4–7 in the liposomes were determined by measuring the absorbance values of [2, 4–7]/[DMPC] at 308, 256, 240, 281 and 282 nm, respectively, after subtracting the scattering of the DMPC liposomes (Fig. 1 and S1†). The dashed lines shown in Fig. 1 represent the extrapolated absorbance values corresponding to the dissolution of 100% of the guests in water. The absorbance values of LMI2, 4–6 lay on the extrapolated lines below 10 mol%. It is noteworthy that similar results have been observed for stilbene (1) and biphenyl (3), neither of which contains a pyridyl moiety. These results therefore suggest that the inclusion of a pyridyl moiety scarcely affected the formation of the LMIGs. In contrast, the absorbance of LMI7 was saturated above 5 mol% (Fig. 1 green circle), indicating that the maximum tolerated ratio of 7 was less than 5 mol%, making it lower than those of the other pyridyl derivatives prepared by the premixing method.
 |
| | Fig. 1 UV-vis absorption spectra of the LMIGs ([2–7]/[DMPC] = 2.5, 5.0 and 20.0 mol%, [2–7] = 0.2 mM). The absorbance (Abs) at λmax versus [G]/[DMPC] in the LMIGs. The λmax values were 308, 253, 256, 240, 281 and 282 nm for LMI2 (black circle), LMI3 (purple circle), LMI4 (red circle), LMI5 (blue circle), LMI6 (orange circle) and LMI7 (green circle), respectively. All of these absorption spectra were obtained by subtracting the light scattering of the DMPC liposomes and were measured in water at 25 °C (1 mm cell). | |
3.2.2 The effect of adding a hydrophilic group to the naphthalene derivatives. Compounds 8–16 are naphthalene derivatives containing a variety of different hydrophilic groups. The absorbance values of LMI8 and LMI9 bearing hydroxy groups were consistent with the extrapolated lines for concentrations of less than 10 mol%, but deviated from the lines at a concentration of 20 mol%. A similar trend was also observed for LMI14 bearing a formyl group (Fig. 2 black, purple and green circles). The absorbance peaks of 20 mol% solutions of LMI8, LMI9 and LMI14 did not show any peak broadening (Fig. S2A, B and F†). Furthermore, no precipitation was observed in these samples. This deviation was therefore attributed to compounds 8, 9 and 14 not being incorporated into the liposomes at 20 mol%, with the compounds being removed by filtration through the membranes (pore size 50 nm) during the extrusion process. In contrast, the absorbance values of LMI10 bearing two naphthol units; LMI11 and LMI13 bearing carboxy groups; and LMI15 and LMI16 bearing amino groups were consistent with the extrapolated lines up to a concentration of 20 mol% (Fig. 2, S2C–E, S3A and S3B†). These results indicated that these guest molecules were well dispersed in water in the presence of liposomes up until a concentration of 20 mol%. Although several attempts were made to solubilize compound 12 bearing two carboxy groups in several organic solvents, the concentrations of 12 were too low to allow for the preparation of LMI12 using the premixing method.
 |
| | Fig. 2 UV-vis absorption spectra of the LMIGs ([8–11 or 13–16]/[DMPC] = 2.5, 5.0 and 20.0 mol%, [8–11 or 13–16] = 0.2 mM). The absorbance (Abs) at λmax versus [G]/[DMPC] in the LMIGs. The λmax values were determined to be 229, 235, 231, 250, 287, 242 and 223 nm for LMI8 (black circle and dashed line), LMI9 (purple circle and dashed line), LMI10 (red circle and dashed line), LMI11 (blue circle and dashed line), LMI13 (orange circle and dashed line), LMI14 (green circle and dashed line), LMI15 (black square and solid line) and LMI16 (red square and solid line), respectively. All of the absorption spectra were obtained by subtracting the light scattering from the DMPC liposomes and were measured in H2O at 25 °C (1 mm cell). [DMPC] = 4.0 mM for 8, 9, 11 and 13–16, [DMPC] = 2.0 mM for 10. | |
3.2.3 The effects of lactone derivatives. To evaluate the effect of adding a lactone moiety, we examined the water-solubilization properties of coumarin (17), 7-methylcoumarine (18) and phthalide (19). The solubilities of compounds 17–19 were determined based on the absorbance of [17–19]/[DMPC] at 320 nm using the procedure described above for compounds 1–16. The absorbance values of LMI17–19 were all consistent with the extrapolated lines for concentrations below 10 mol%, suggesting that most of the molecules of 17–19 had successfully dissolved in the water (Fig. 3 and S3C–E†).
 |
| | Fig. 3 UV-vis absorption spectra of the LMIGs ([17–19]/[DMPC] = 2.5, 5.0 and 20.0 mol%, [17–19] = 0.2 mM). The absorbance (Abs) at λmax versus [G]/[DMPC] in the LMIGs. The λmax values were determined to be 278, 278, 231 and 239 nm for LMI17 (black circle), LMI18 (red circle) and LMI19 (blue circle), respectively. All of the absorption spectra were obtained by subtracting the light scattering from the DMPC liposomes and were measured in H2O at 25 °C (1 mm cell). [DMPC] = 4.0 mM. | |
3.3. Determination of formation of LMIGs by 1H NMR analyses
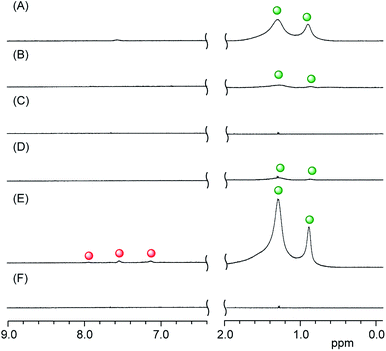
3.3.1 Formation of lipid membrane-incorporated aromatic compounds bearing pyridyl groups. Compounds 1–6 dissolved in water in the presence of the DMPC liposomes at a concentration of [1–6]/[DMPC] = 10 mol%, making it possible to confirm the formation of the LMIGs by 1H NMR analysis at 10 mol% (Fig. 4B–G and S4†). The 1H NMR spectra of the LMIGs did not contain any peaks that could be assigned to DMPC or the guest molecules in the LMIGs because of the extreme broadening of the signals following the formation of the liposomes (Fig. 4A). The appearance of any peaks assignable to DMPC or the guest molecules would therefore indicate that the DMPC and guest molecules had been released from the liposomes. The peak intensities in Fig. 4A–G and S4† were normalized relative to the value of DMSO (0.4 mM), which was adopted as an internal standard. As shown in Fig. 4B, no signals were observed for DMPC or 1, indicating that all of the molecules of DMPC and 1 had formed LMI1 (Scheme 1A). Similar results were observed for compounds 2 and 3 (Fig. 4C and D). Fig. 4E–G shows that the addition of compounds 4–6 to a solution of DMPC liposomes did not lead to the appearance of any new peaks in the range of 0.8–1.4 ppm, which were attributed to the alkyl chains of free DMPC, but did result in the appearance of several new peaks assignable to 4–6. These results clearly indicated that several molecules of 4–6 had leaked from the lipid membranes into the bulk aqueous environment, where they had dissolved without the assistance of DMPC (Scheme 1D). The leakage percentages of 4–6 were determined to be 5, 59 and 36%, respectively, based on the peak intensities of compounds 4–6 relative to the DMSO peak.
 |
| | Fig. 4 Partial 1H NMR spectra of (A) the DMPC liposome, (B) LMI1, (C) LMI2, (D) LMI3, (E) LMI4, (F) LMI5 and (G) LMI6 in D2O at 25 °C ( : free guest molecule). [1–6]/[DMPC] = 10.0 mol%, [DMPC] = 4.0 mM, [DMSO] = 0.4 mM. : free guest molecule). [1–6]/[DMPC] = 10.0 mol%, [DMPC] = 4.0 mM, [DMSO] = 0.4 mM. | |
 |
| | Scheme 1 Schematic illustration of (A) stable LMIG [category (i)], (B) the precipitation of the guest molecules released from LMIG [category (ii)], (C) the formation of small aggregates between the guest molecules released from LMIG and the lipids [category (iii)] and (D) the dissolution of the guest molecules released from LMIG in water [category (iv)]. | |
3.3.2 Formation of lipid membrane-incorporated naphthalene derivatives. With the exception of 12, compounds 8–16 dissolved in D2O containing DMPC at a concentration of less than 10 mol%, allowing their 1H NMR spectra to be measured at 10 mol%. As shown in Fig. 5C, D, H, S5 and S6,† several new broad peaks were observed in the 1H NMR spectra of LMI10, 11 and 16 bearing hydroxy, carboxy or amino groups in the range of 0.8–1.4 ppm, which were attributed to the alkyl chains of DMPC. If the DMPC liposomes remained as large hollow particles with a diameter greater than 70 nm, then these broad peaks would not be observed because of the extreme broadening of the signals. These results therefore suggested that some of the DMPC liposomes had collapsed, with some of DMPC lipids forming small aggregates such as micelles or very small liposomes with 10, 11 or 16 (Scheme 1C). Given that the peak intensities of DMPC are greatly dependent on the size of its aggregates, it is not possible to determine the concentration DMPC in a small aggregate based on the peak intensities in the range of 0.8–1.4 ppm. However, because larger peaks were observed in the range of 0.8–1.4 ppm for LMI10, compound 10 was adjudged to have destabilized the DMPC liposomes to a much greater extent than compounds 11 and 16. In contrast, the 1H NMR spectra of compounds 8, 9 and 13–15 contained no peaks assignable to DMPC in the range of 0.8–1.4 ppm, despite being similar in structure to compound 11 (Fig. 5A, B and E–G). Furthermore, no peaks assignable to 8, 9 and 13–15 were observed by 1H NMR analysis in the range of 7.0–9.0 ppm, which suggested that the vast majority of these compounds had been successfully incorporated into the lipid membranes. This result therefore indicated that LMI8, LMI9 and LMI13–15 were stable once they had formed (Scheme 1A).
 |
| | Fig. 5 Partial 1H NMR spectra of (A) LMI8, (B) LMI9, (C) LMI10, (D) LMI11, (E) LMI13, (F) LMI14, (G) LMI15 and (H) LMI16 in D2O at 25 °C ( : DMPC lipid). [8–11 or 13–16]/[DMPC] = 10.0 mol%, [DMPC] = 4.0 mM, [DMSO] = 0.4 mM. The insets show the region of 0.5–2.0 ppm. : DMPC lipid). [8–11 or 13–16]/[DMPC] = 10.0 mol%, [DMPC] = 4.0 mM, [DMSO] = 0.4 mM. The insets show the region of 0.5–2.0 ppm. | |
3.3.3 Formation of lipid membrane-incorporated lactone derivatives. As shown in Fig. 6A–C and S7,† the 1H NMR spectra of LMI17–19 contained several new peaks in the range of 6.4–8.2 ppm, which were assignable to compounds 17–19. In contrast, no new peaks were observed in the range of 0.8–1.4 ppm for compounds 17–19. These results suggested that the DMPC liposomes did not collapse in the presence of these guest compounds and that several molecules of 17–19 had leaked from the lipid membranes to the bulk water, where they dissolved without any assistance from DMPC. The leakage percentages of 17–19 were determined to be 49, 40 and 100%, respectively, based on the peak intensities of compounds 17–19 relative to DMSO (Table 1). The leakage percentage of 18 (40%) was lower than that of 17 (49%), most likely because of the hydrophobicity of the methyl group in 18. In contrast, the leakage percentage of 19 (100%) was much higher than those of 17 and 18. The reason for this difference will be discussed in detail below in the section 3.4.3.
 |
| | Fig. 6 Partial 1H NMR spectra of (A) LMI17, (B) LMI18 and (C) LMI19 in D2O at 25 °C ( : free guest molecule). [17–19]/[DMPC] = 10.0 mol%, [DMPC] = 4.0 mM, [DMSO] = 0.4 mM. : free guest molecule). [17–19]/[DMPC] = 10.0 mol%, [DMPC] = 4.0 mM, [DMSO] = 0.4 mM. | |
Table 1 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow and leakage percentage values for compounds 1–19
Pow and leakage percentage values for compounds 1–19
| Compound |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Powa Powa |
Leakage percentageb |
Category |
| DMPC liposome |
DPPC liposome |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow was calculated using version 11.02 of the Advanced Chemistry Development Software (ACD/labs., Toronto, Canada). The leakage values were determined by 1H NMR spectroscopy. Some of the guest molecules precipitated out for [G]/[DMPC] = 10 mol%. The guest molecules did not dissolve in any organic solvents. Pow was calculated using version 11.02 of the Advanced Chemistry Development Software (ACD/labs., Toronto, Canada). The leakage values were determined by 1H NMR spectroscopy. Some of the guest molecules precipitated out for [G]/[DMPC] = 10 mol%. The guest molecules did not dissolve in any organic solvents. |
| 1 |
4.83 |
0 |
|
(i) |
| 2 |
3.34 |
0 |
|
(i) |
| 3 |
3.98 |
0 |
|
(i) |
| 4 |
2.59 |
5 |
0 |
(iv) and (i) |
| 5 |
1.20 |
59 |
72 |
(iv) and (iv) |
| 6 |
1.28 |
36 |
15 |
(iv) and (iv) |
| 7 |
2.86 |
—c |
|
(ii) |
| 8 |
2.71 |
0 |
|
(i) |
| 9 |
1.98 |
0 |
|
(i) |
| 10 |
4.44 |
0 |
0 |
(iii) and (i) |
| 11 |
3.13 |
0 |
2 |
(iii) and (iv) |
| 12 |
2.04 |
—d |
|
(ii) |
| 13 |
2.39 |
0 |
|
(i) |
| 14 |
2.87 |
0 |
|
(i) |
| 15 |
2.17 |
0 |
|
(i) |
| 16 |
0.88 |
0 |
2 |
(iii) and (iv) |
| 17 |
1.39 |
49 |
46 |
(iv) and (iv) |
| 18 |
1.85 |
40 |
37 |
(iv) and (iv) |
| 19 |
0.99 |
100 |
29 |
(iv) and (iv) |
3.4. Abundance of the guest molecules in lipid membranes
These phenomena can be classified into four different categories when [G]/[lipid] = 10 mol%, including (i) most of the guest molecules form stable LMIGs (Scheme 1A); (ii) some of the guest molecules precipitate from solution (Scheme 1B); (iii) some of the guest molecules form small aggregates such as micelles or very small liposomes with the lipids (Scheme 1C); and (iv) some of the guest molecules are released from the lipid membranes (Scheme 1D). For category (i), the guest molecules would have a high affinity for the acyl chains of the lipids. In contrast, the guest molecules in category (ii) would have a lower affinity for the acyl chains of the lipids than those in category (i). The fullerenes and porphyrins prepared in the current study using the premixing method would therefore belong in category (ii).11,23 However, given that LMIfullerenes and LMIporphyrins can also be prepared using an exchange method with cyclodextrins,11–26 the guest molecules in category (ii) would be incorporated into the lipid membranes if they were included in cyclodextrins. Categories (iii) and (iv) will be discussed in detail in the following sections.
3.4.1 Formation of small aggregates. Compounds 10, 11 and 16 were included in category (iii) (Table 1). Although the guest molecules in category (iii) labilized the LMIGs in the same way as those belonging to categories (ii) and (iv), the guest molecules in the labilized LMIGs were not solely released from the liposomes but were also included in the small aggregate released by the fission of the liposomes. We initially considered that the dipole moments of the guest molecules could play an important role in determining whether they formed aggregates. However, this hypothesis was discarded when we established that 14 (3.0 D) formed no aggregate despite having larger dipole moment than 11 (2.5 D). Although the underlying mechanism of this process currently remains unclear, there are similarities between these phenomena and the formation of micelles following the surfactant-mediated collapse of liposomes under aqueous conditions.35,36 Furthermore, it has been reported that the incorporation of some guest molecules can lead to a decrease in the diameter of the liposome.37,38 Such decreases in the diameter can be caused by local increases in the curvature of the liposome, which occur as a geometric consequence of the incorporation of a cone-like guest molecules into a phospholipid bilayer. We analyzed the LMIGs in category (iii) by dynamic light scattering (DLS) and cryogenic transmission electron microscopy (cryo-TEM) analyses to determine whether the small aggregates formed in these cases were micelles or very small liposomes. The results of the DLS measurements revealed that the hydrodynamic diameters (Dhy) and polydispersity indices of the liposomes barely changed for [G]/[lipid] = 10 mol% (Table 2). This result suggested that while some of the liposomes had collapsed to form small aggregates most of them remained unchanged. This idea was also supported by the following two experimental results. Although we were unable to accurately calculate the peak intensities of DMPC because of considerable peak broadening, the percentages of DMPC in the small aggregates were estimated to be approximately 25, 7 and 8% for 10, 11 and 16, respectively. These results showed that less than 25% of the liposome had broken down to form small aggregates. The cryo-TEM images revealed that fewer liposomes were observed in the presence of 10 ([10]/[DMPC] = 10 mol%) than there were in the absence of 10 (Fig. 7). Furthermore, the size of the liposomes remained almost constant regardless of the presence or absence of 10 (Fig. 7). We supposed that the aggregates were too small to be observed by cryo-TEM.
Table 2 Hydrodynamic diameters (Dhy) of the DMPC liposome, as determined by dynamic light-scattering at 25 °C in the absence and the presence of the guest molecules or surfactant
| Guest molecule |
[G]/[DMPC]/mol% |
Hydrodynamic diameter/nm |
Polydispersity index |
| — |
0 |
88 |
0.12 |
| 10 |
10 |
83 |
0.11 |
| 10 |
30 |
101 |
0.16 |
| 11 |
10 |
79 |
0.18 |
| 16 |
10 |
91 |
0.10 |
| TX-100 |
10 |
135 |
0.30 |
| TX-100 |
30 |
74 |
0.32 |
| TX-100 |
1000 |
8 |
0.27 |
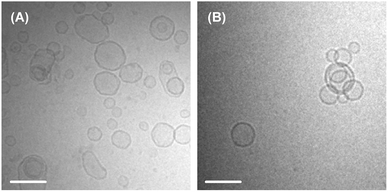 |
| | Fig. 7 Cryo TEM images of (A) DMPC liposome and (B) LMI10. [10]/[DMPC] = 30.0 mol%, [DMPC] = 1.0 mM. | |
It is well known that liposomes can collapse and form micelles after the addition of a large amount of a suitable lysing agent (e.g., ethanol) or a surfactant under aqueous conditions.39,40 If the model described in category (iii) is correct, we would expect compounds 10, 11 and 16 to act as surfactants. With this in mind, we compared the effects of adding the nonionic surfactant triton-X 100 (TX-100) to LMI10. For [TX-100]/[DMPC] = 10 mol%, 1H NMR analysis revealed the slightly broadened peaks of TX-100, as shown in Fig. 8B and S8B† (purple circles). When the concentration was increased to 30 mol%, 1H NMR analysis showed that there was a considerable increase in the broadening of the peaks in the range of 0.8–1.4 ppm (Fig. 8C and S8C,† green mark). These peaks were attributed to the formation of small aggregates composed of DMPC and TX-100. The broad peaks assignable to the DMPC lipids were similar to those observed in LMI10, LMI11 and LMI16. However, DLS measurements revealed that the Dhy value of the liposomes decreased slightly from 88 to 74 nm following the addition of TX-100 (30 mol%) (Table 2), which was similar to the changes observed for guest molecules 10, 11 and 16 in category (iii). In contrast, much sharper peaks were observed for both TX-100 and DMPC in the range of 0.8–1.4 ppm after the TX-100-mediated collapse of all of the liposomes ([TX-100]/[DMPC] = 1000 mol%) (Fig. 8D and S8D†). Furthermore, after all of the DMPC liposomes had collapsed based on their peak intensities, the Dhy value was determined to be 8 nm by DLS (Table 2), which clearly indicated that all the DMPC molecules had formed very small micelles with TX-100. The broadening of the peaks in Fig. 8C indicated that the small aggregates formed between DMPC and TX-100 at 30 mol% were larger than the micelles formed by TX-100. The results observed for TX-100 strongly support the formation of small aggregates in LMI10, LMI11 and LMI16 (Scheme 1C). The small aggregates formed by the addition of 10 were also not complete small micelles but rather large micelles or small liposomes. In fact, for [10]/[DMPC] = 30 mol%, the peaks in the range of 0.8–1.4 ppm became sharper than those observed at 10 mol%. Unfortunately, it was not possible to investigate the effects of adding more 10 because it was not all incorporated in the lipid membranes for [10]/[DMPC] > 50 mol% by UV-vis absorption analysis.
 |
| | Fig. 8 Partial 1H NMR spectra of (A) TX-100, DMPC-TX-100 mixtures with [TX-100]/[DMPC] = (B) 10.0, (C) 30.0 and (D) 1000 mol% and (E) LMI10 with [10]/[DMPC] = 30.0 mol% in D2O at 25 °C ( : guest molecule, : guest molecule,  : TX-100 and : TX-100 and  : DMPC lipid in the small aggregates). [DMPC] = 1.0 mM, [DMSO] = 0.1 mM. : DMPC lipid in the small aggregates). [DMPC] = 1.0 mM, [DMSO] = 0.1 mM. | |
3.4.2 Inhibiting the formation of small aggregates. We considered that the formation of small aggregates could be attributed to the lability of the DMPC liposomes. These spectra were measured at 25 °C, which is above the phase transition temperature (Tm) between the gel and liquid-crystal phases of the DMPC liposomes (Tm = 23 °C). To determine the effect in the gel phase with the tight packing of the hydrophobic chains, we carried out 1H NMR measurement involving LMI10 at a temperature below the Tm of LMI10. As shown in Fig. 5C, 9A, B, S9A and S9B,† the 1H NMR spectrum of an LMI10 solution recorded at 20 °C showed that the peaks observed in the range of 0.8–1.4 ppm were not as broad as those observed at temperatures of 25 and 30 °C (>Tm). The result indicated that while the gel phase of the DMPC liposomes could prevent the formation of small aggregates, it could not do so completely. Based on this result we prepared LMI10 consisting of DPPC, which has been reported to form much more stable liposomes than DMPC. Fig. 9C–E and S9C–S9E† show the 1H NMR spectra of LMI10 consisting of DPPC liposomes at 25, 37 (body temperature) and 50 °C, respectively. The intensities of the broadened peaks in the range of 0.8–1.4 ppm increased with increasing temperature, indicating that the temperature has a much greater influence on the formation of the small aggregates than Tm. After cooling at 25 °C (Fig. 9F and S9F†), the spectrum returned to what it was before heating (Fig. 9C), indicating that the formation of the small self-aggregates was a reversible process. In contrast, LMI11 and LMI16 gave new broadened peaks in the range of 7.0–9.0 ppm (Fig. 10D, E, S10D and S10E†). These results indicated that the more stable DPPC liposomes had prevented the formation of small aggregates to a much greater extent than the DMPC liposomes, whereas 11 and 16 were released from the DPPC liposomes and consequently included in category (iv). Furthermore, the peaks observed in the 1H NMR spectra of LMI5, 6 and LMI17–19 in the ranges of 7.0–9.0 ppm did not disappear after cooling at 25 °C (Fig. 10B, C, F–H, S10B, C and F–H†), indicating that 5, 6 and 17–19 remained in category (iv) even by use of DPPC liposomes. In contrast, the broadened peaks observed in the 1H NMR spectra of LMI4 in the ranges of 0.8–1.4 and 7.0–9.0 ppm disappeared after cooling at 25 °C (Fig. 10A and S10A†), indicating that most of the guest molecules formed stable LMIGs in the same manner as those in category (i).
 |
| | Fig. 9 Partial 1H NMR spectra of LMI10 consisting of DMPC in D2O at (A) 30 °C and (B) 20 °C and LMI10 consisting of DPPC in D2O at (C) 25 °C, (D) 37 °C and (E) 50 °C followed by cooling to (F) 25 °C ( : guest molecule and : guest molecule and  : DMPC lipids in the small aggregates). [10]/[lipid] = 10.0 mol%, [lipid] = 4.0 mM, [DMSO] = 0.4 mM. : DMPC lipids in the small aggregates). [10]/[lipid] = 10.0 mol%, [lipid] = 4.0 mM, [DMSO] = 0.4 mM. | |
 |
| | Fig. 10 Partial 1H NMR spectra of (A) LMI4, (B) LMI5, (C) LMI6, (D) LMI11, (E) LMI16, (F) LMI17 (G) LMI18 and (H) LMI19 consisting of DPPC in D2O at 25 °C ( : guest molecule). [4, 5, 6, 11, 16, 17, 18 or 19]/[DPPC] = 10.0 mol%, [DPPC] = 4.0 mM, [DMSO] = 0.4 mM. : guest molecule). [4, 5, 6, 11, 16, 17, 18 or 19]/[DPPC] = 10.0 mol%, [DPPC] = 4.0 mM, [DMSO] = 0.4 mM. | |
3.4.3 Relationship between the abundance of the compound in the lipid membrane and its log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Pow value. The log
Pow value. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow value of a guest molecule is usually defined as the ratio of its concentrations in the two phases of a biphasic mixture composed of 1-octanol and water. Although the log
Pow value of a guest molecule is usually defined as the ratio of its concentrations in the two phases of a biphasic mixture composed of 1-octanol and water. Although the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow values of guest molecules are usually determined experimentally, it is also possible to estimate these value using several commercially available computer programs.31
Pow values of guest molecules are usually determined experimentally, it is also possible to estimate these value using several commercially available computer programs.31For log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow > 1.9, most of the compounds belonged to category (i) (Fig. 11, black) and formed stable LMIGs. Although compound 4 (log
Pow > 1.9, most of the compounds belonged to category (i) (Fig. 11, black) and formed stable LMIGs. Although compound 4 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow > 2.59) was category (iv) (Fig. 11, red), the leakage percentage of 4 was 5%. For log
Pow > 2.59) was category (iv) (Fig. 11, red), the leakage percentage of 4 was 5%. For log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow < 1.9, most of the compounds belonged to category (iv) (Fig. 11, red) except for compound 16. Thus, although this value cannot be used to explain the behaviors of all of the guest compounds, the threshold for the leakage of the guest molecules was determined to be approximately 1.9. The threshold toward the DMPC and DPPC liposomes is almost same (Fig. 11, open red squares and triangle).
Pow < 1.9, most of the compounds belonged to category (iv) (Fig. 11, red) except for compound 16. Thus, although this value cannot be used to explain the behaviors of all of the guest compounds, the threshold for the leakage of the guest molecules was determined to be approximately 1.9. The threshold toward the DMPC and DPPC liposomes is almost same (Fig. 11, open red squares and triangle).
 |
| | Fig. 11 Comparison of the leakage percentages of the guest molecules with the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow values of compounds 1–6 (circle), 8–16 (square) and 17–19 (triangle); DMPC (closed) and DPPC (open) liposomes; categories (i) (black), (iii) (blue) and (iv) (red), in D2O at 25 °C. [G]/[lipid] = 10.0 mol%, [lipid] = 4.0 mM, [DMSO] = 0.4 mM. Pow values of compounds 1–6 (circle), 8–16 (square) and 17–19 (triangle); DMPC (closed) and DPPC (open) liposomes; categories (i) (black), (iii) (blue) and (iv) (red), in D2O at 25 °C. [G]/[lipid] = 10.0 mol%, [lipid] = 4.0 mM, [DMSO] = 0.4 mM. | |
4. Conclusions
LMIGs can be classified into four different categories, including (i) systems where most of the guest molecules have been incorporated into liposomes to form stable LMIGs; (ii) systems were some of the guest molecules precipitate from solution; (iii) systems were some of the guest molecules form small self-aggregates with lipids; and (iv) systems were some of the guest molecules leak from the lipid membranes and dissolve in the bulk water. Compounds 7 and 12 were included in category (ii) together with the fullerenes and porphyrins in the LMIGs that were prepared by the premixing method. In the premixing method, thin membranes are formed following the concentration of an organic solution of the lipids and guest molecules. The guest molecules included in category (ii) therefore formed larger self-aggregates of the guest molecules than any of the other guest molecules because of their poor solubility in organic solvents. Compounds belonging to categories (i), (iii) and (iv) could be distinguished based on their 1H NMR spectra because all of the peaks belonging to the guest molecules and the lipids disappeared completely in their LMIGs. Compounds 1–3, 8, 9 and 13–15 were included in category (i) in the DMPC liposomes and formed stable LMIGs. All of these compounds had relatively high log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow values (>1.9). Although compound 4 had similarly high log
Pow values (>1.9). Although compound 4 had similarly high log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow value (2.59), it showed some leakage (leakage percentage = 5%). In contrast, it was difficult to categorize compounds 10, 11 and 16, which were ultimately included in category (iii). These behaviors of these systems appeared to be influenced by a local increase in the curvature as a geometric consequence of the incorporation of cone-like guest molecules into the phospholipid bilayers. Compounds 5, 6 and 17–19 were all included in category (iv) with relatively low log
Pow value (2.59), it showed some leakage (leakage percentage = 5%). In contrast, it was difficult to categorize compounds 10, 11 and 16, which were ultimately included in category (iii). These behaviors of these systems appeared to be influenced by a local increase in the curvature as a geometric consequence of the incorporation of cone-like guest molecules into the phospholipid bilayers. Compounds 5, 6 and 17–19 were all included in category (iv) with relatively low log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow values (<1.9). The border between categories (i) and (iv) was defined by log
Pow values (<1.9). The border between categories (i) and (iv) was defined by log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow values in the range of 1.9–2.0 towards DMPC and DPPC liposomes. Further work towards better defining these categories is currently underway in our laboratories using several other guest molecules and lipids.
Pow values in the range of 1.9–2.0 towards DMPC and DPPC liposomes. Further work towards better defining these categories is currently underway in our laboratories using several other guest molecules and lipids.
Acknowledgements
This work was supported by the Japanese Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (B) (Grant No. JP16H04133) and a Grant-in-Aid for Challenging Exploratory Research (Grant No. JP16K13982). The authors would like to express their deepest gratitude to Ms S. Fujita, Graduate School of Materials Science, Nara Institute of Science and Technology for providing technical assistance with the cryo-TEM observations.
Notes and references
- A. D. Bangham, Prog. Biophys. Mol. Biol., 1968, 18, 29–36 CrossRef CAS PubMed.
- G. Sessa and G. Weissmann, J. Lipid Res., 1968, 9, 310–318 CAS.
- Y. Kaneda, Adv. Drug Delivery Rev., 2000, 43, 197–205 CrossRef CAS PubMed.
- T. M. Allen and P. R. Cullis, Science, 2004, 303, 1818–1822 CrossRef CAS PubMed.
- V. P. Torchilin, Nat. Rev. Drug Discovery, 2005, 4, 145–160 CrossRef CAS PubMed.
- H. I. Pass, J. Natl. Cancer Inst., 1993, 85, 443–456 CrossRef CAS PubMed.
- T. J. Dougherty, C. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, J. Natl. Cancer Inst., 1998, 90, 889–905 CrossRef CAS PubMed.
- K. Lang, J. Mosinger and D. M. Wagnerová, Coord. Chem. Rev., 2004, 248, 321–350 CrossRef CAS , and references therein.
- P. L. Felgner, T. R. Gadek, M. Holm, R. Roman, H. W. Chan, M. Wenz, J. P. Northrop, G. M. Ringold and M. Danielsen, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 7413–7417 CrossRef CAS.
- Liposomes: A Practical Approach, ed. V. P. Torchilin and W. Weissig, Oxford University Press, Oxford, 2nd edn, 2003 Search PubMed.
- A. Ikeda, S. Hino, T. Mae, Y. Tsuchiya, K. Sugikawa, M. Tsukamoto, K. Yasuhara, H. Shigeto, H. Funabashi, A. Kuroda and M. Akiyama, RSC Adv., 2015, 5, 105279–105287 RSC.
- A. Ikeda, Chem. Rec., 2016, 16, 249–260 CrossRef CAS PubMed.
- A. Ikeda, T. Sato, K. Kitamura, K. Nishiguchi, Y. Sasaki, J. Kikuchi, T. Ogawa, K. Yogo and T. Takeya, Org. Biomol. Chem., 2005, 3, 2907–2909 CAS.
- A. Ikeda, Y. Doi, K. Nishiguchi, K. Kitamura, M. Hashizume, J. Kikuchi, K. Yogo, T. Ogawa and T. Takeya, Org. Biomol. Chem., 2007, 5, 1158–1160 CAS.
- A. Ikeda, Y. Doi, M. Hashizume, J. Kikuchi and T. Konishi, J. Am. Chem. Soc., 2007, 129, 4140–4141 CrossRef CAS PubMed.
- Y. Doi, A. Ikeda, M. Akiyama, M. Nagano, T. Shigematsu, T. Ogawa, T. Takeya and T. Nagasaki, Chem.–Eur. J., 2008, 14, 8892–8897 CrossRef CAS PubMed.
- A. Ikeda, T. Sue, M. Akiyama, K. Fujioka, T. Shigematsu, Y. Doi, J. Kikuchi, T. Konishi and R. Nakajima, Org. Lett., 2008, 10, 4077–4080 CrossRef CAS PubMed.
- A. Ikeda, M. Nagano, M. Akiyama, M. Matsumoto, S. Ito, M. Mukai, M. Hashizume, J. Kikuchi, K. Katagiri, T. Ogawa and T. Takeya, Chem.–Asian J., 2009, 4, 199–205 CrossRef CAS PubMed.
- A. Ikeda, Y. Kawai, J. Kikuchi and M. Akiyama, Chem. Commun., 2010, 46, 2847–2849 RSC.
- A. Ikeda, M. Akiyama, T. Ogawa and T. Takeya, ACS Med. Chem. Lett., 2010, 1, 115–119 CrossRef CAS PubMed.
- A. Ikeda, Y. Kawai, J. Kikuchi, M. Akiyama, E. Nakata, Y. Uto and H. Hori, Org. Biomol. Chem., 2011, 9, 2622–2627 CAS.
- A. Ikeda, K. Kiguchi, T. Shigematsu, K. Nobusawa, J. Kikuchi and M. Akiyama, Chem. Commun., 2011, 47, 12095–12097 RSC.
- A. Ikeda, T. Hida, T. Iizuka, M. Tsukamoto, J. Kikuchi and K. Yasuhara, Chem. Commun., 2014, 50, 1288–1291 RSC.
- A. Ikeda, S. Hino, T. Mae, Y. Tsuchiya, K. Sugikawa, M. Tsukamoto, K. Yasuhara, H. Shigeto, H. Funabashi, A. Kuroda and M. Akiyama, RSC Adv., 2015, 5, 105279–105287 RSC.
- A. Ikeda, M. Mori, K. Kiguchi, K. Yasuhara, J. Kikuchi, K. Nobusawa, M. Akiyama, M. Hashizume, T. Ogawa and T. Takeya, Chem.–Asian J., 2012, 7, 605–613 CrossRef CAS PubMed.
- A. Ikeda, Chem. Rec., 2016, 16, 249–260 CrossRef CAS PubMed.
- Y. Katz and J. M. Diamond, J. Membr. Biol., 1974, 17, 69–86 CrossRef CAS PubMed.
- S. T. Burns and M. G. Khaledi, J. Pharm. Sci., 2002, 91, 1601–1612 CrossRef CAS PubMed.
- A. Klamt, U. Huniar, S. Spycher and J. Keldenich, J. Phys. Chem. B, 2008, 112, 12148–12157 CrossRef CAS PubMed.
- S. Endo, B. I. Escher and K.-U. Goss, Environ. Sci. Technol., 2011, 45, 5912–5921 CrossRef CAS PubMed.
- The values of log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow were calculated using Advanced Chemistry Development Software V11.02 (ACD/labs.).
Pow were calculated using Advanced Chemistry Development Software V11.02 (ACD/labs.). - A. Ikeda, T. Hida, T. Nakano, S. Hino, K. Nobusawa, M. Akiyama and K. Sugikawa, Chem. Lett., 2014, 43, 1551–1553 CrossRef CAS.
- K. Nakazawa, M. Hishida, S. Nagatomo, Y. Yamamura and K. Saito, Chem. Lett., 2014, 43, 1352–1354 CrossRef CAS.
- A. Ikeda, S. Hino, K. Ashizawa, K. Sugikawa, J. Kikuchi, M. Tsukamoto and K. Yasuhara, Org. Biomol. Chem., 2015, 13, 6175–6182 CAS.
- J. Lasch, Biochim. Biophys. Acta, 1995, 1241, 269–292 CrossRef.
- H. Heerklotz, Q. Rev. Biophys., 2008, 41, 205–264 CrossRef CAS PubMed.
- M. A. Perillo and D. A. Garcia, Colloids Surf., B, 2001, 20, 63–72 CrossRef CAS PubMed.
- Y. Cohen, H. Weitman, M. Afri, R. Yanus, S. Rudnick, Y. Talmon, J. Schmidt, P. Aped, S. Shatz, B. Ehrenberg and A. A. Frimer, J. Liposome Res., 2012, 22, 306–318 CrossRef CAS PubMed.
- S. M. K. Davidson and S. L. Regen, Chem. Rev., 1997, 97, 1269–1279 CrossRef CAS PubMed , and references therein.
- A. Mueller and D. F. O'Brien, Chem. Rev., 2002, 102, 727–757 CrossRef CAS PubMed , and references therein.
Footnote |
| † Electronic supplementary information (ESI) available: UV-vis absorption and 1H NMR spectra. See DOI: 10.1039/c6ra18635a |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.  *,
Kengo Ashizawa,
Yuki Tsuchiya,
Masafumi Ueda and
Kouta Sugikawa
*,
Kengo Ashizawa,
Yuki Tsuchiya,
Masafumi Ueda and
Kouta Sugikawa
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Klipw) of substituted phenols and several other compounds.27–31 Although this value can be useful for evaluating the affinity of a guest molecule for lipid and cell membranes, it still remains difficult to determine whether guest molecules have been successfully incorporated into liposomes at high concentrations. In this paper, we have 1H NMR spectroscopy to determine the leakage percentages of guest molecules from liposomes. The results of this study have shown that LMIGs were not only labilized by the guest molecules towards leaking their guest molecules into water but that they also formed small self-aggregates with lipids. Furthermore, we have investigated the relationship between the equilibrium and the octanol–water partition coefficient (log
Klipw) of substituted phenols and several other compounds.27–31 Although this value can be useful for evaluating the affinity of a guest molecule for lipid and cell membranes, it still remains difficult to determine whether guest molecules have been successfully incorporated into liposomes at high concentrations. In this paper, we have 1H NMR spectroscopy to determine the leakage percentages of guest molecules from liposomes. The results of this study have shown that LMIGs were not only labilized by the guest molecules towards leaking their guest molecules into water but that they also formed small self-aggregates with lipids. Furthermore, we have investigated the relationship between the equilibrium and the octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow) for some model small π-compounds (1–19) and confirmed the threshold values log
Pow) for some model small π-compounds (1–19) and confirmed the threshold values log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow for these systems.28
Pow for these systems.28
 : free guest molecule). [17–19]/[DMPC] = 10.0 mol%, [DMPC] = 4.0 mM, [DMSO] = 0.4 mM.
: free guest molecule). [17–19]/[DMPC] = 10.0 mol%, [DMPC] = 4.0 mM, [DMSO] = 0.4 mM.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow and leakage percentage values for compounds 1–19
Pow and leakage percentage values for compounds 1–19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Powa
Powa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow was calculated using version 11.02 of the Advanced Chemistry Development Software (ACD/labs., Toronto, Canada).b The leakage values were determined by 1H NMR spectroscopy.c Some of the guest molecules precipitated out for [G]/[DMPC] = 10 mol%.d The guest molecules did not dissolve in any organic solvents.
Pow was calculated using version 11.02 of the Advanced Chemistry Development Software (ACD/labs., Toronto, Canada).b The leakage values were determined by 1H NMR spectroscopy.c Some of the guest molecules precipitated out for [G]/[DMPC] = 10 mol%.d The guest molecules did not dissolve in any organic solvents.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) Pow value. The log
Pow value. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow value of a guest molecule is usually defined as the ratio of its concentrations in the two phases of a biphasic mixture composed of 1-octanol and water. Although the log
Pow value of a guest molecule is usually defined as the ratio of its concentrations in the two phases of a biphasic mixture composed of 1-octanol and water. Although the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow values of guest molecules are usually determined experimentally, it is also possible to estimate these value using several commercially available computer programs.31
Pow values of guest molecules are usually determined experimentally, it is also possible to estimate these value using several commercially available computer programs.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow > 1.9, most of the compounds belonged to category (i) (Fig. 11, black) and formed stable LMIGs. Although compound 4 (log
Pow > 1.9, most of the compounds belonged to category (i) (Fig. 11, black) and formed stable LMIGs. Although compound 4 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow > 2.59) was category (iv) (Fig. 11, red), the leakage percentage of 4 was 5%. For log
Pow > 2.59) was category (iv) (Fig. 11, red), the leakage percentage of 4 was 5%. For log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow < 1.9, most of the compounds belonged to category (iv) (Fig. 11, red) except for compound 16. Thus, although this value cannot be used to explain the behaviors of all of the guest compounds, the threshold for the leakage of the guest molecules was determined to be approximately 1.9. The threshold toward the DMPC and DPPC liposomes is almost same (Fig. 11, open red squares and triangle).
Pow < 1.9, most of the compounds belonged to category (iv) (Fig. 11, red) except for compound 16. Thus, although this value cannot be used to explain the behaviors of all of the guest compounds, the threshold for the leakage of the guest molecules was determined to be approximately 1.9. The threshold toward the DMPC and DPPC liposomes is almost same (Fig. 11, open red squares and triangle).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow values (>1.9). Although compound 4 had similarly high log
Pow values (>1.9). Although compound 4 had similarly high log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow value (2.59), it showed some leakage (leakage percentage = 5%). In contrast, it was difficult to categorize compounds 10, 11 and 16, which were ultimately included in category (iii). These behaviors of these systems appeared to be influenced by a local increase in the curvature as a geometric consequence of the incorporation of cone-like guest molecules into the phospholipid bilayers. Compounds 5, 6 and 17–19 were all included in category (iv) with relatively low log
Pow value (2.59), it showed some leakage (leakage percentage = 5%). In contrast, it was difficult to categorize compounds 10, 11 and 16, which were ultimately included in category (iii). These behaviors of these systems appeared to be influenced by a local increase in the curvature as a geometric consequence of the incorporation of cone-like guest molecules into the phospholipid bilayers. Compounds 5, 6 and 17–19 were all included in category (iv) with relatively low log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow values (<1.9). The border between categories (i) and (iv) was defined by log
Pow values (<1.9). The border between categories (i) and (iv) was defined by log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow values in the range of 1.9–2.0 towards DMPC and DPPC liposomes. Further work towards better defining these categories is currently underway in our laboratories using several other guest molecules and lipids.
Pow values in the range of 1.9–2.0 towards DMPC and DPPC liposomes. Further work towards better defining these categories is currently underway in our laboratories using several other guest molecules and lipids.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pow were calculated using Advanced Chemistry Development Software V11.02 (ACD/labs.).
Pow were calculated using Advanced Chemistry Development Software V11.02 (ACD/labs.).


















