Macroscopic and microscopic insight into the mutual effects of europium(III) and phosphate on their interaction with graphene oxide†
Huan Xuab,
Jun Huc,
Xuemei Ren*b and
Guang Li*ad
aSchool of Physics and Materials Science, Anhui University, Hefei 230601, P. R. China. E-mail: liguang1971@ahu.edu.cn
bInstitute of Plasma Physics, Chinese Academy of Sciences, P.O. Box 1126, Hefei 230031, P. R. China. E-mail: renxm1985@163.com; Fax: +86-551-65591310; Tel: +86-551-65593308
cSchool of Electronic Engineering, Dongguan University of Technology, Dongguan 523808, P. R. China
dAnhui Key Laboratory of Information Materials and Devices, Hefei 230601, P. R. China
First published on 30th August 2016
Abstract
Graphene oxide (GO) has been proved to be very efficient for radionuclide enrichment. However, its potential performance for binary sorbate systems containing both a radionuclide and phosphate receives little attention. In this study, the mutual effects of Eu(III) and phosphate on their co-removal processes by GO as a function of contact time, pH and temperature were investigated in detail. The results show that the presence of phosphate can enhance not only the removal amount of Eu(III) but also the removal kinetics of Eu(III) on GO surfaces, and vice versa. Both the external mass transfer and diffusion of solute inside GO are important for the Eu(III) and phosphate co-removal rate. Evaluation of the thermodynamic parameters (ΔG0 < 0, ΔH0 > 0 and ΔS0 > 0) indicates the co-removal process is endothermic and spontaneous. The results of X-ray photoelectron spectroscopy (XPS) and zeta potential (ZP) analyses indicate that the oxygen-containing functional groups on GO surfaces play an important role in the co-removal process. This study provides a quantitative investigation of Eu(III) and phosphate with GO, which can facilitate the assessment of GO as a potential adsorbent for Eu(III) and phosphate simultaneous cleanup.
1. Introduction
Radionuclide contamination generated from the development of the nuclear industry poses serious threats to aquatic habitats and living organisms due to its potential toxic and carcinogenic effects.1,2 The radionuclides with high mobility can be accumulated through the food chain even at very low concentrations, ultimately becoming a part of animals and human beings.3 Over the past few decades, approximately 10.7 kg of lanthanides and actinides are present in each ton of used nuclear fuel.4 Therefore, the highly efficient elimination and long-term storage of the long-lived radionuclides from large volumes of nuclear wastewater have currently become an issue of great environmental concern. However, the high radioactivity and biological toxicity limit some lanthanides and actinides (such as 236Np, 241Am, 243Am, 243Cm, 244Pu, etc.) for use for experimental investigation in laboratories.5 Europium (Eu(III)) has been used as a chemical analog for the trivalent lanthanides and actinides such as Am(III) and Cm(III).6,7 Phosphate is deemed as a double-edged nutrient for both the growth of organisms and eutrophication of rivers and lakes in most ecosystems.8 Phosphate may be present in large amounts as a result of TBP (tributyl phosphate used in the PUREX process in reprocessing plants) degradation by radiolysis.9 Therefore, radionuclide and phosphate are likely to co-exist in the effluent of reprocessing plants. The development of a facile and efficient approach to simultaneously remove both Eu(III) and phosphate from nuclear wastewater is urgent and crucial.Owing to the excellent water-solubility, large specific surface area (theoretical value of 2620 m2 g−1), and enriched oxygenated functional groups (such as carboxyl, epoxy and hydroxyl), graphene oxide (GO) has been reported of highly efficient sorption capacities for radionuclides.10–12 Zhao et al.10 firstly applied GO to remove U(VI) from aqueous solutions, and found the sorption process was mainly dominated by inner-sphere surface complexation. Sun et al.11 studied the interaction of Eu(III) with GO by extended X-ray absorption fine structure (EXAFS) spectroscopy, and found the sorption capacity of Eu(III) on GO was 1.15 mmol g−1 at pH 6.0. Romanchuk et al.12 reported the prepared GO using improved Hummers' method demonstrated high sorption affinity towards actinides (i.e., U(VI), Sr(II), Am(III) and Eu(III)). However, few studies focused on the application of GO as the potential adsorbent in the binary sorbate systems containing both radionuclides and phosphate.13 Phosphate can readily form complexes with many radionuclides and interact with scavengers, affecting their surface charge and overall reactivity.14 Both of these may potentially affect the sorption of Eu(III). Therefore, investigations that incorporate phosphate into the co-removal process may have more realistic implications for Eu(III) cleanup in nuclear wastewater than those without co-adsorbing anions.
The purposes of this study are: (1) to synthesize GO and apply it as the adsorbent for the co-removal of Eu(III) and phosphate; (2) to elaborate the effects of contact time, pH and temperature on the co-removal process; and (3) to investigate the possible sorption mechanisms of Eu(III) and phosphate on GO. This study provides new insights of GO as the potential adsorbent for Eu(III) and phosphate co-removal by adopting batch techniques, which can broaden the applicability of graphene derivatives in simultaneous radionuclide and oxyanion pollution cleanup.
2. Material and method
2.1. Chemicals and adsorbent preparation
Expandable graphite (<20 μm) was provided from Qingdao Tianhe Graphite Co., Ltd (China). The expandable graphite was used as the raw material instead of flake graphite to ensure more uniform oxidization.15,16 The chemicals including H2SO4, NaNO3, KMnO4, H2O2, NaBH4, NaH2PO4·2H2O, NaCl, HCl and NaOH were purchased from Sinopharm Chemical Reagent Co., Ltd. All chemicals used in the experiments were of analytical grade and used directly without further purification. Milli-Q water (18.2 MΩ cm−1) was used to prepare the solutions throughout the experiments.The GO was synthesized via the chemical oxidation of expandable graphite by employing the modified Hummers' method.17 The stock GO suspension had a dark brown color and did not form coagulation even after 6 months of aging time.10,18 The homogeneous dispersion property of GO in aqueous solutions will make the oxygen-containing functional groups on GO surfaces freely available to form strong surface complexes with Eu(III).
2.2. Characterization
The obtained GO samples were characterized by scanning electron microscopy (SEM), Fourier transform infrared (FT-IR) spectroscopy, Raman spectroscopy, and UV-vis absorbance spectroscopy techniques. The GO samples before and after the co-removal process of Eu(III) and phosphate were characterized by energy dispersive spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS) and zeta potential (ZP) techniques. The SEM image was obtained using a JEOL JSM-6700F instrument operated at the beam energy of 15.0 kV. The FT-IR measurement was recorded in pressed KBr pellets (Aldrich, 99%, analytical reagent) by using a PerkinElmer Spectrum 100 system spectrometer at room temperature. The Raman spectrum was conducted using a LabRam HR Raman spectrometer with excitation at 514.5 nm for 10 s by Ar+ laser to avoid overheating of the GO. The absorbance of the GO sample in aqueous solution (∼25 mg L−1) was characterized using UV-vis spectroscopy (UV-2550, PerkinElmer). The ZP values of the GO samples in the absence and presence of Eu(III) or/and phosphate were recorded as a function of pH using a Zetasizer Nano-ZS90 Instrument (Malvern Co., U.K.) at T = 298 K. The XPS spectra were conducted using a VG Scientific ESCALAB Mark II spectrometer equipped with two ultrahigh vacuum chambers. The peak energies of XPS spectra were corrected with C 1s peak at 284.6 eV as a reference.16 The deconvolution of C 1s and O 1s lines were performed using XPSPEAK41 program after subtraction of the background (Shirley baseline correction).2.3. Batch sorption experiments
The sorption of Eu(III) and/or phosphate on GO were carried out by batch experiments at T = 293, 313 and 333 K. In order to achieve homogeneous dispersion, the stock suspension of GO was sonicated for 15 min in an ultrasonic bath before use. Appropriate amounts of NaCl (0.1 mol L−1), Eu(III) (0.5 mmol L−1) and/or phosphate (0.5 mmol P L−1) stock solutions and the stock suspension of GO (100 mg L−1) were added into a 10 mL polyethylene test tube to achieve the desired concentrations of different components. After adding the above components and controlling the final volume of suspension in the test tube to 6 mL, the initial concentrations of GO, NaCl, Eu(III) and phosphate in the test tube were 25 mg L−1, 0.01 mol L−1, 0.05 mmol L−1 and 0.05 mmol P L−1, respectively. The desired pH value of suspension was adjusted by adding negligible volumes of 0.1/0.01 mol L−1 HCl or NaOH solution. Additionally, in order to find the difference between individual and co-existing systems, single Eu(III)/phosphate sorption as a function of contact time was also investigated at T = 293 K. After the suspension was shaken for 24 h, the solid and liquid phases were separated by the centrifugation at 9500 rpm for 30 min, and then the supernatant was filtered through a 0.22 μm membrane filter. The concentration of Eu(III) in the supernatant was measured by monitoring the Eu(III)–chlorophosphonazo complex by spectrophotometry at a wavelength of 632 nm based on GB/T 18116.2-2008. The concentration of phosphate in the supernatant was measured by monitoring the blue phosphate–molybdate complex at 700 nm based on GB/T 11893-1989. The co-existing phosphate will not affect the quantitative detection result of Eu(III), and vice versa (Fig. S1 in ESI†).The sorption percentage (%), distribution coefficient (Kd), and the amounts of Eu(III) or phosphate adsorbed on GO (Cs) are calculated from the following equations, respectively:
 | (1) |
 | (2) |
 | (3) |
3. Results and discussion
3.1. Characterization
Fig. 1a shows the SEM image of the obtained GO samples. The wrinkled and aggregated ripples on GO surfaces are observed, which is consistent with the previous report.19 The oxygen-containing functional groups on GO surfaces are characterized by FT-IR technique (Fig. 1b). The absorption bands at 1077, 1624, 1725 and 3423 cm−1 correspond to C–O bonds, sp2 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, C
C bonds, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations and –OH stretching vibrations, respectively.19 The FT-IR spectrum indicates that a large amount of oxygen-containing functional groups (i.e., carbonyl, carboxyl, epoxy and hydroxyl) present on GO surfaces. In the Raman spectrum (Fig. 1c), the G band at ∼1580 cm−1 is associated with the vibration of sp2 carbon atoms in a graphitic 2D hexagonal lattice, and the D band at ∼1350 cm−1 is related to the vibration of sp3 carbon atoms of defects. The D band is resulted from the structural defection created by the attachment of epoxy and hydroxyl functional groups on the carbon basal plane.16 The strong intensities of G and D bands are consistent with previous GO characterizations.10,17 According to the UV-absorbance spectrum (Fig. 1d), the maximum peak at 227 nm corresponds to π–π stretching vibrations of aromatic C
O stretching vibrations and –OH stretching vibrations, respectively.19 The FT-IR spectrum indicates that a large amount of oxygen-containing functional groups (i.e., carbonyl, carboxyl, epoxy and hydroxyl) present on GO surfaces. In the Raman spectrum (Fig. 1c), the G band at ∼1580 cm−1 is associated with the vibration of sp2 carbon atoms in a graphitic 2D hexagonal lattice, and the D band at ∼1350 cm−1 is related to the vibration of sp3 carbon atoms of defects. The D band is resulted from the structural defection created by the attachment of epoxy and hydroxyl functional groups on the carbon basal plane.16 The strong intensities of G and D bands are consistent with previous GO characterizations.10,17 According to the UV-absorbance spectrum (Fig. 1d), the maximum peak at 227 nm corresponds to π–π stretching vibrations of aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds.16 The high resolution scans for C 1s and O 1s are shown in Fig. 1e and f, respectively. The C 1s XPS spectrum of GO presents two separate peaks due to the high percentage of oxygenated functionalities. The deconvolution of C 1s spectrum indicates the presence of C–C at 284.8 eV, C–O groups at 287.1 eV, and –COOH groups at 288.1 eV, which can be comparable to the previous reports.18,19 The deconvolution of O 1s spectrum exhibits two peaks with binding energies of 531.8 and 532.9 eV, which are assigned to C
C bonds.16 The high resolution scans for C 1s and O 1s are shown in Fig. 1e and f, respectively. The C 1s XPS spectrum of GO presents two separate peaks due to the high percentage of oxygenated functionalities. The deconvolution of C 1s spectrum indicates the presence of C–C at 284.8 eV, C–O groups at 287.1 eV, and –COOH groups at 288.1 eV, which can be comparable to the previous reports.18,19 The deconvolution of O 1s spectrum exhibits two peaks with binding energies of 531.8 and 532.9 eV, which are assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O, respectively.18 From the C 1s and O 1s XPS spectra, the considerable degree oxygen-containing functional groups on GO surfaces are C–O, C
O and C–O, respectively.18 From the C 1s and O 1s XPS spectra, the considerable degree oxygen-containing functional groups on GO surfaces are C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O
O and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O, which have potentiality to form strong complexes with radionuclides.
C–O, which have potentiality to form strong complexes with radionuclides.
 | ||
| Fig. 1 Characterizations of GO: (a) SEM image, (b) FT-IR spectrum, (c) Raman spectrum, (d) UV-vis absorbance spectrum and high resolution XPS spectra for (e) C 1s and (f) O 1s. | ||
3.2. Eu(III) sorption
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
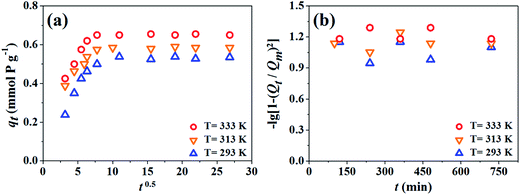
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the sorption process increases much more rapidly in the initial 30 min of contact time at the lowest temperature (T = 293 K), and then maintains a high level until the sorption process achieves equilibrium within 1 h. Fig. 2 also shows that the sorption capacity of GO for Eu(III) in the presence of phosphate is clearly influenced by temperature. The higher temperature, the more Eu(III) sorption, suggesting that higher temperature favors Eu(III) sorption in ternary system containing Eu(III), phosphate and GO. In general, the sorption process of Eu(III) is rapid and 2 h is enough to achieve sorption equilibrium. Based on the sorption kinetic data, a shaking time of 24 h is chosen in the following experiments to ensure that the sorption process could achieve complete equilibrium.
1), the sorption process increases much more rapidly in the initial 30 min of contact time at the lowest temperature (T = 293 K), and then maintains a high level until the sorption process achieves equilibrium within 1 h. Fig. 2 also shows that the sorption capacity of GO for Eu(III) in the presence of phosphate is clearly influenced by temperature. The higher temperature, the more Eu(III) sorption, suggesting that higher temperature favors Eu(III) sorption in ternary system containing Eu(III), phosphate and GO. In general, the sorption process of Eu(III) is rapid and 2 h is enough to achieve sorption equilibrium. Based on the sorption kinetic data, a shaking time of 24 h is chosen in the following experiments to ensure that the sorption process could achieve complete equilibrium.
The migration process and overall sorption rate of the sorbate at water–solid interface are controlled by the surface characteristics and diffusion resistances of the solid particles.21 Therefore, the utilization of a suitable kinetic model can provide useful information in determining the underlying mechanism during the entire sorption process. The experimental kinetic data of Eu(III) sorption are simulated by the pseudo-first-order and pseudo-second-order models using the following equations, respectively:22,23
ln(Qm − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qm − k′t Qm − k′t
| (4) |
 | (5) |
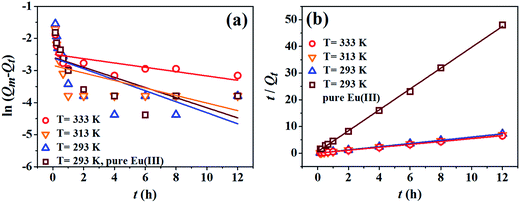
The fitting results of pseudo-first-order and pseudo-second-order models are shown in Fig. 3a and b, respectively, and the corresponding kinetic parameters are listed in Table 1. As shown in Table 1, the pseudo-second-order model (eqn (5)) simulates the kinetic data better than the pseudo-first-order model (eqn (4)) with higher correlation coefficients and the calculated equilibrium values (Qmc) closer to the experimental ones (Qme), which highlights the chemisorption-controlled mechanism.24 The values of Qmc (1.66 mmol g−1) and k′′ (4.56 g mmol−1 h−1) in the presence of phosphate are much higher than the ones in the absence of phosphate, respectively, suggesting the presence of phosphate can enhance not only the removal amount of Eu(III) but also the removal kinetics of Eu(III) on GO surfaces.
| Parameters | Eu(III) | Phosphate | |||||
|---|---|---|---|---|---|---|---|
| 293b K | 293 K | 313 K | 333 K | 293 K | 313 K | 333 K | |
| a Qmc is the sorption capacity at equilibrium calculated from different kinetic models, Qme is the experimental value.b Parameters of single Eu(III) sorption simulated by different kinetic models. | |||||||
| Pseudo-first-order model | |||||||
| k′ (h−1) | 0.16 | 0.17 | 0.12 | 0.07 | 0.18 | 0.15 | 0.15 |
| Qmc (mmol g−1) | 0.08 | 0.07 | 0.06 | 0.08 | 0.11 | 0.08 | 0.07 |
| R2 | 0.489 | 0.391 | 0.282 | 0.361 | 0.428 | 0.384 | 0.302 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Pseudo-second-order model | |||||||
| k′′ (g mmol−1 h−1) | 2.59 | 4.56 | 6.65 | 4.85 | 1.50 | 2.79 | 3.14 |
| Qmc (mmol g−1) | 0.26 | 1.66 | 1.75 | 1.84 | 0.54 | 0.59 | 0.66 |
| R2 | 0.995 | 0.998 | 0.999 | 0.999 | 0.997 | 0.999 | 0.998 |
| Qme (mmol g−1) | 0.27 | 1.67 | 1.78 | 1.88 | 0.56 | 0.61 | 0.67 |
(1) The kinetic data can be used in determining the time required to achieve sorption equilibrium.
(2) Sorption rate calculated from the kinetic study can be used to deduce predictive models for batch experiments.
(3) Kinetic studies can be used in understanding the effects of variables on the sorption of solutes.
Generally, the sorption kinetics is controlled by the film diffusion step, particle diffusion step, and sequestration on the active sites via sorption, complexation or intraparticle precipitation.26 The last step is supposed to be very rapid and can be recognized as negligible. For the purpose of identifying the slowest step in sorption process, it is necessary to differentiate film diffusion step from particle diffusion step. Sorption rate usually depends on parameters such as metallic ions concentration, structural properties of adsorbent (specific area, porosity, particle size, etc.), the properties of metallic ions (ionic radius, coordination numbers, and speciation), interaction between active sites of adsorbent and metallic ions.25,26 If intraparticle diffusion plays the dominant role in sorption process, it should be well fitted by the relationship between Qt and the square root of time (t0.5) as follows:25
 | (6) |
As shown in Fig. 4a, a nonlinear distribution of points with two distinct portions is observed. The double nature of the plots (curved and linear) obtained from Eu(III) sorption on GO indicates the existence of intraparticle diffusion. According to intraparticle diffusion model,26 if there is a linear relationship between Qt and t0.5, it will indicate that intraparticle/pore diffusion is the rate-limiting step in sorption process. However, the plots shown in Fig. 4a contrast the prediction of intraparticle diffusion model, suggesting that intraparticle/pore diffusion may participate in the sorption process but is not the single rate-limiting step. The initial curved part of plots is attributed to film diffusion, the linear part, to intraparticle diffusion.25
Because of the multistep nature of these plots, the linear portions are modeled by Urano and Tachikawa model, which is shown as follows:27
 | (7) |
According to Urano and Tachikawa model,27 if the plots lie in a linear form and pass through origin, then the rate-limiting step of sorption process is internal diffusion, and vice versa. From Fig. 4b, it is obvious that only the plot of Eu(III) sorption on GO in the presence of phosphate at 313 K is linear but does not pass through the origin, suggesting that the rate-limiting step is film diffusion. Other plots are not linear, suggesting that either internal diffusion or film diffusion is not the rate-limiting step. It can be assumed that both the external mass transfer and the diffusion of solute inside GO are important for the single Eu(III) sorption rate and for Eu(III) and phosphate co-removal rate.
 | (8) |
| Cs = KFCen | (9) |
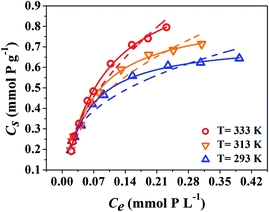
The parameters calculated from these two models are listed in Table 2. It is clear that the Langmuir model fits the sorption isotherms better than Freundlich model with higher correlation coefficients, suggesting surface sites are homogeneous and that monolayer sorption is occurring for Eu(III) sorption in the binary and ternary systems.32 Based on the Langmuir model, the Cs,max values for Eu(III) sorption are the highest at T = 333 K and the lowest at T = 293 K, suggesting that higher temperature is more conducive to Eu(III) sorption. In the Freundlich model, n is lower than 1, suggesting that a nonlinear sorption occurs on GO surfaces. These results indicate that Eu(III) sorption should be associated with chemical sorption rather than physical sorption, which is consistent with the result of kinetic study. As compared with the adsorbents previously reported in other literatures (Table 3), GO possesses higher Eu(III) sorption capacity in the presence of phosphate than most of the common adsorbents.6,7,11,12,33
| Parameters | Eu(III) | Phosphate | ||||
|---|---|---|---|---|---|---|
| 293 K | 313 K | 333 K | 293 K | 313 K | 333 K | |
| Langmuir isotherm fit | ||||||
| Cs,max (mmol g−1) | 2.51 | 2.93 | 3.38 | 0.73 | 0.86 | 1.09 |
| KL (L mg−1) | 41.33 | 50.50 | 67.80 | 19.95 | 16.05 | 11.60 |
| R2 | 0.996 | 0.996 | 0.998 | 0.996 | 0.996 | 0.998 |
| RL | 0.93 | 0.98 | 0.93 | 0.95 | 0.95 | 0.93 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Freundlich isotherm fit | ||||||
| n | 0.39 | 0.37 | 0.34 | 0.34 | 0.40 | 0.51 |
| KF (mmol1−n Ln g−1) | 4.57 | 5.39 | 6.21 | 0.95 | 1.23 | 1.75 |
| R2 | 0.946 | 0.945 | 0.938 | 0.945 | 0.960 | 0.974 |
| Adsorbent | Experimental condition | Cs,max (mmol g−1) | Ref. |
|---|---|---|---|
| Eu(III) | |||
| Bentonite–PAAm composites | pH = 3.5, T = 293 K | 0.28 | 6 |
| GO@TiP nanocomposites | pH = 5.5, T = 298 K | 0.42 | 7 |
| Ordered mesoporous carbon | pH = 4.0, T = 298 K | 0.91 | 33 |
| GO | pH = 5.0, T = 298 K | 0.76 | 12 |
| GO | pH = 6.0, T = 298 K | 1.15 | 11 |
| GO | pH = 4.0, T = 293 K, in the presence of 0.05 mmol L−1 phosphate | 2.51 | This work |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Phosphate | |||
| Iron oxide tailings | pH = 6.6–6.8, T = 293 K | 0.26 | 39 |
| MgO/CaO-loaded bentonite | pH = 7.5, T = 298 K | 0.37 | 40 |
| Magnetic Fe–Zr binary oxide | pH = 4.0, T = 298 K | 0.44 | 41 |
| γ-Al2O3 | pH = 5.5, T = 298 K, in the presence of 0.09 mmol L−1 Cu(II) | 0.14 | 29 |
| Natroalunite microtubes | pH = 7.0, T = 298 K, in the presence of 0.2 mmol L−1 Cd(II) | 0.72 | 32 |
| GO | pH = 4.0, T = 293 K, in the presence of 0.05 mmol L−1 Eu(III) | 0.73 | This study |
In order to examine whether the sorption is favorable or not, the essential characteristics of the Langmuir model can be expressed in terms of a dimensionless constant separation factor or equilibrium parameter, RL, which is given by the following equation:32
 | (10) |
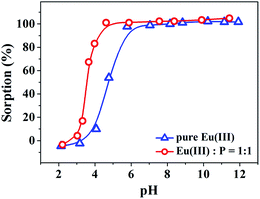
According to Hameed et al.,34 the value of RL indicates the sorption to be unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1) or irreversible (RL = 0). The relevant RL parameters listed in Table 2 fall in the range of 0–1, suggesting that the sorption of Eu(III) on GO is favorable, and Eu(III) ions tend to remain binding on GO surfaces.34
ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 K0
| (11) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd versus Ce and extrapolating Ce to zero.
Kd versus Ce and extrapolating Ce to zero.The standard enthalpy change (ΔH0) and standard entropy change (ΔS0) are calculated from the following equation:
 | (12) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 versus 1/T are −ΔH0/R and ΔS0/R, respectively.
K0 versus 1/T are −ΔH0/R and ΔS0/R, respectively.
Linear plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd versus Ce and ln
Kd versus Ce and ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 versus 1/T for the sorption of Eu(III) retained by GO in the presence of phosphate (0.05 mmol P L−1) at different temperatures are shown in Fig. 7a and b, respectively. Thermodynamic parameters of Eu(III) sorption are listed in Table 4. The negative ΔG0 values (−8.81, −12.46 and −16.61 kJ mol−1 at 293, 313 and 333 K, respectively) indicate that the co-removal process on GO surfaces is favorable and spontaneous. The positive ΔH0 value (48.22 kJ mol−1) confirms the co-removal process to be endothermic, which is consistent with the results of kinetic and sorption isotherm studies. According to the previous studies,35,36 the positive value of ΔS0 reflects the increased disorder at the water–solid interface with some structural changes in the adsorbent and sorbate during the sorption process, and an affinity of the adsorbent towards sorbate. In our case, the value of ΔS0 is 194.40 J mol−1 K−1, which reflects the high affinity of GO towards Eu(III) in the presence of phosphate, and also indicates an increase in the degree of freedom of the adsorbed species.
K0 versus 1/T for the sorption of Eu(III) retained by GO in the presence of phosphate (0.05 mmol P L−1) at different temperatures are shown in Fig. 7a and b, respectively. Thermodynamic parameters of Eu(III) sorption are listed in Table 4. The negative ΔG0 values (−8.81, −12.46 and −16.61 kJ mol−1 at 293, 313 and 333 K, respectively) indicate that the co-removal process on GO surfaces is favorable and spontaneous. The positive ΔH0 value (48.22 kJ mol−1) confirms the co-removal process to be endothermic, which is consistent with the results of kinetic and sorption isotherm studies. According to the previous studies,35,36 the positive value of ΔS0 reflects the increased disorder at the water–solid interface with some structural changes in the adsorbent and sorbate during the sorption process, and an affinity of the adsorbent towards sorbate. In our case, the value of ΔS0 is 194.40 J mol−1 K−1, which reflects the high affinity of GO towards Eu(III) in the presence of phosphate, and also indicates an increase in the degree of freedom of the adsorbed species.
| Temperature | Eu(III) | Phosphate | ||||
|---|---|---|---|---|---|---|
| 293 K | 313 K | 333 K | 293 K | 313 K | 333 K | |
| ΔG0 (kJ mol−1) | −8.81 | −12.46 | −16.61 | −4.26 | −6.03 | −8.42 |
| ΔH0 (kJ mol−1) | 48.22 | 26.07 | ||||
| ΔS0 (J mol−1 K−1) | 194.40 | 103.21 | ||||
3.3. Phosphate sorption
Two kinetic model simulations (i.e., pseudo-first-order and pseudo-second-order models) for phosphate sorption on GO in the presence of Eu(III) at different temperatures are shown in Fig. 9a and b, respectively, and the corresponding kinetic parameters are listed in Table 1. The correlation coefficients of the pseudo-second-order rate equation (eqn (5)) are close to 1, which is higher than those of the pseudo-first-order model (eqn (4)). Meanwhile, the Qmc values of phosphate sorption calculated from the pseudo-second-order model are closer to Qme. Both suggest that the pseudo-second-order model simulates the kinetic data better, highlighting the chemisorption-controlled mechanism.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd versus Ce and ln
Kd versus Ce and ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 versus 1/T for the sorption of phosphate retained by GO in the presence of Eu(III) (0.05 mmol L−1) at different temperatures are shown in Fig. 13a and b, respectively. Thermodynamic parameters of phosphate sorption are listed in Table 4. With the increasing temperature, the calculated ΔG0 values decrease, with values of −4.26, −6.03 and −8.42 kJ mol−1 at 293, 313 and 333 K, respectively. The negative ΔG0 values indicate that the co-removal process is favorable and spontaneous. ΔH0 and ΔS0 are calculated to be 26.07 kJ mol−1 and 103.21 J mol−1 K−1, respectively. The positive ΔH0 value further confirms the sorption of phosphate to be endothermic, which is consistent with the results of kinetic and sorption isotherm studies.
K0 versus 1/T for the sorption of phosphate retained by GO in the presence of Eu(III) (0.05 mmol L−1) at different temperatures are shown in Fig. 13a and b, respectively. Thermodynamic parameters of phosphate sorption are listed in Table 4. With the increasing temperature, the calculated ΔG0 values decrease, with values of −4.26, −6.03 and −8.42 kJ mol−1 at 293, 313 and 333 K, respectively. The negative ΔG0 values indicate that the co-removal process is favorable and spontaneous. ΔH0 and ΔS0 are calculated to be 26.07 kJ mol−1 and 103.21 J mol−1 K−1, respectively. The positive ΔH0 value further confirms the sorption of phosphate to be endothermic, which is consistent with the results of kinetic and sorption isotherm studies.
3.4. Interaction mechanisms of Eu(III) and phosphate with GO
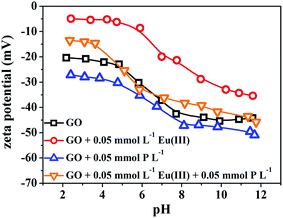
In order to further investigate the interaction mechanism of Eu(III) and phosphate with GO, the obtained GO samples before and after the co-removal process (i.e., denoted as GO and GO + Eu + P, respectively) are characterized by XPS technique. Fig. 14a shows the XPS survey spectra of GO and GO + Eu + P. One can see that carbon and oxygen are the predominant elements on GO surfaces, and the peaks of Eu 3d and P 2p are observed in the survey spectrum of GO + Eu + P.20 Fig. 14b shows the high resolution scans for O 1s of GO and GO + Eu + P. The shift of binding energy and the decrease of peak area of O 1s spectra are observed after the co-removal process, suggesting that Eu(III) and phosphate are chemically adsorbed on GO surfaces.20 The high resolution scans for Eu 3d5/2 and P 2p of GO + Eu + P are shown in Fig. 14c and d, respectively. Fig. 14e and f show the deconvolutions of C 1s and O 1s high resolution scans before and after the co-removal process. One can see that there are significant differences in C 1s spectra associated with oxygen-containing functional groups such as C–O in carbonyl (286.6 eV) and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carboxylic (287.0 eV) groups between GO and GO + Eu + P (both in intensities and peak positions). The O 1s spectra of GO and GO + Eu + P differ from each other significantly both in the shape and maximum position. The XPS survey spectra and high resolution scans for C 1s and O 1s clearly demonstrate that the co-removal process of Eu(III) and phosphate is related to the oxygen-containing functional groups on GO surfaces.
O in carboxylic (287.0 eV) groups between GO and GO + Eu + P (both in intensities and peak positions). The O 1s spectra of GO and GO + Eu + P differ from each other significantly both in the shape and maximum position. The XPS survey spectra and high resolution scans for C 1s and O 1s clearly demonstrate that the co-removal process of Eu(III) and phosphate is related to the oxygen-containing functional groups on GO surfaces.
4. Conclusions
In this study, batch experiments including contact time, pH and temperature were employed to study the performance of GO in the co-removal of Eu(III) and phosphate. The sorption characteristics of GO for Eu(III) and phosphate can be affected obviously by the presence of the other one, and the co-removal process is highly dependent on pH and temperature. The sorption kinetics of Eu(III) and phosphate follow the pseudo-second-order model, and the sorption isotherms can be well fitted by the Langmuir isotherm model. The standard thermodynamic parameters indicate that the co-removal process of Eu(III) and phosphate on GO surfaces is endothermic and spontaneous. The sorption enhancement of Eu(III) in the presence of phosphate will minimize the potential risk of Eu(III) to water quality. Findings of this study highlight the potential utility of GO as the attractive adsorbent for Eu(III) and phosphate co-removal in nuclear wastewater treatment.Acknowledgements
Financial supports from Anhui Provincial Natural Science Foundation (1608085QB44 and 1508085MB29), and the National Natural Science Foundation of China (21307135, 21225730, 41273134, 21377132, 91326202) are acknowledged.References
- Y. B. Sun, J. X. Li and X. K. Wang, Geochim. Cosmochim. Acta, 2014, 140, 621–643 CrossRef CAS.
- Y. B. Sun, Z. Y. Wu, X. X. Wang, C. C. Ding, W. C. Cheng, S. H. Yu and X. K. Wang, Environ. Sci. Technol., 2016, 50, 4459–4467 CrossRef CAS PubMed.
- T. Wen, X. L. Wu, M. C. Liu, Z. H. Xing, X. K. Wang and A. W. Xu, Dalton Trans., 2014, 43, 7464–7472 RSC.
- J. A. Shusterman, H. E. Mason, J. Bowers, A. Bruchet, E. C. Uribe, A. B. Kersting and H. Nitsche, ACS Appl. Mater. Interfaces, 2015, 7, 20591–20599 CAS.
- X. X. Wang, S. B. Yang, W. Q. Shi, J. X. Li, T. Hayat and X. K. Wang, Environ. Sci. Technol., 2015, 49, 11721–11728 CrossRef CAS PubMed.
- X. X. Wang, S. S. Lu, L. Chen, J. X. Li, S. Y. Dai and X. K. Wang, J. Radioanal. Nucl. Chem., 2015, 306, 497–505 CrossRef CAS.
- C. R. Li, Y. Huang and Z. Lin, J. Mater. Chem. A, 2014, 2, 14979–14985 CAS.
- H. B. Yin, M. X. Han and W. Y. Tang, Chem. Eng. J., 2016, 285, 671–678 CrossRef CAS.
- Z. Y. Chen, Q. Jin, Z. J. Guo, G. Montavon and W. S. Wu, Chem. Eng. J., 2014, 256, 61–68 CrossRef CAS.
- G. X. Zhao, T. Wen, X. Yang, S. B. Yang, J. L. Liao, J. Hu, D. D. Shao and X. K. Wang, Dalton Trans., 2012, 41, 6182–6188 RSC.
- Y. B. Sun, Q. Wang, C. L. Chen, X. L. Tan and X. K. Wang, Environ. Sci. Technol., 2012, 46, 6020–6027 CrossRef CAS PubMed.
- A. Y. Romanchuk, A. S. Slesarev, S. N. Kalmykov, D. V. Kosynkin and J. M. Tour, Phys. Chem. Chem. Phys., 2013, 15, 2321–2327 RSC.
- S. Bachmaf, B. P. Friedrich and B. J. Merkel, Radiochim. Acta, 2008, 96, 359–366 CrossRef CAS.
- S. R. Wang, W. L. Yi, S. W. Yang, X. C. Jin, G. D. Wang and F. C. Wu, Appl. Geochem., 2011, 26, 286–292 CrossRef CAS.
- X. L. Li, X. R. Wang, L. Zhang, S. W. Lee and H. J. Dai, Science, 2008, 319, 1229–1232 CrossRef CAS PubMed.
- Y. B. Sun, S. B. Yang, C. C. Ding, Z. X. Jin and W. C. Cheng, RSC Adv., 2015, 5, 24886–24892 RSC.
- G. X. Zhao, L. Jiang, Y. D. He, J. X. Li, H. L. Dong, X. K. Wang and W. P. Hu, Adv. Mater., 2011, 23, 3959–3963 CrossRef CAS PubMed.
- H. Xu, G. Li, J. Li, C. L. Chen and X. M. Ren, J. Mol. Liq., 2016, 213, 58–68 CrossRef CAS.
- Y. B. Sun, S. B. Yang, Y. Chen, C. C. Ding, W. C. Cheng and X. K. Wang, Environ. Sci. Technol., 2015, 49, 4255–4262 CrossRef CAS PubMed.
- C. C. Ding, W. C. Cheng, Y. B. Sun and X. K. Wang, Dalton Trans., 2014, 43, 3888–3896 RSC.
- S. T. Yang, P. F. Zong, X. M. Ren, Q. Wang and X. K. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 6891–6900 CAS.
- Y. S. Ho and G. Mckay, Process Saf. Environ. Prot., 1998, 76, 332–340 CrossRef CAS.
- Y. S. Ho and G. Mckay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- W. C. Song, X. X. Wang, Q. Wang, D. D. Shao and X. K. Wang, Phys. Chem. Chem. Phys., 2015, 17, 398–406 RSC.
- V. C. T. Costodes, H. Fauduet, C. Porte and A. Delacroix, J. Hazard. Mater., 2003, 105, 121–142 CrossRef.
- C. O. Ijagbemi, M. H. Baek and D. S. Kim, J. Hazard. Mater., 2009, 166, 538–546 CrossRef CAS PubMed.
- K. Urano and H. Tachikawa, Ind. Eng. Chem. Res., 1991, 30, 1897–1899 CrossRef CAS.
- P. N. Pathak and G. R. Choppin, Radiochim. Acta, 2007, 95, 267–273 CAS.
- X. M. Ren, S. T. Yang, X. L. Tan, C. L. Chen, G. D. Sheng and X. K. Wang, J. Hazard. Mater., 2012, 237, 199–208 CrossRef PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- H. M. A. Frendlich, J. Phys. Chem., 1906, 57, 385 Search PubMed.
- H. Xu, B. S. Zhu, X. M. Ren, D. D. Shao, X. L. Tan and C. L. Chen, J. Hazard. Mater., 2016, 314, 249–259 CrossRef CAS PubMed.
- D. Dayananda, V. R. Sarva, S. V. Prasad, J. Arunachalam and N. N. Ghosh, Chem. Eng. J., 2014, 248, 430–439 CrossRef CAS.
- B. H. Hameed, A. T. M. Din and A. L. Ahmad, J. Hazard. Mater., 2007, 141, 819–825 CrossRef CAS PubMed.
- S. B. Wang and Z. H. Zhu, J. Hazard. Mater., 2006, 136, 946–952 CrossRef CAS PubMed.
- V. S. Mane, I. D. Mall and V. C. Srivastava, Dyes Pigm., 2007, 73, 269–278 CrossRef CAS.
- K. A. Krishnan and A. Haridas, J. Hazard. Mater., 2008, 152, 527–535 CrossRef CAS PubMed.
- T. P. Moss, J. X. Wang, S. Jones, E. May, D. Olive, Z. R. Dai, M. Zavarin, A. B. Kersting, D. Y. Zhao and H. Nitsche, J. Mater. Chem. A, 2014, 2, 11209–11221 Search PubMed.
- L. Zeng, X. M. Li and J. D. Liu, Water Res., 2004, 38, 1318–1326 CrossRef CAS PubMed.
- J. R. Li, L. Zhu, J. F. Tang, K. Qin, G. Li and T. H. Wang, RSC Adv., 2016, 6, 23252–23259 RSC.
- F. Long, J. L. Gong, G. M. Zeng, L. Chen, X. Y. Wang, J. H. Deng, Q. Y. Niu, H. Y. Zhang and X. R. Zhang, Chem. Eng. J., 2011, 171, 448–455 CrossRef CAS.
- I. Carabante, M. Grahn, A. Holmgren, J. Kumpiene and J. Hedlund, Environ. Sci. Technol., 2012, 46, 13152–13159 CrossRef CAS PubMed.
- X. M. Ren, Q. Y. Wu, H. Xu, D. D. Shao, X. L. Tan, W. Q. Shi, C. L. Chen, J. X. Li, T. Hayat and X. K. Wang, Environ. Sci. Technol., 2016 DOI:10.1021/acs.est.6b02934.
Footnote |
| † Electronic supplementary information (ESI) available: Working curves simulation (Fig. S1), SEM image and EDS patterns of GO (Fig. S2), and saturation index (Fig. S3). See DOI: 10.1039/c6ra18629g |
| This journal is © The Royal Society of Chemistry 2016 |