NIR fluorescent DCPO glucose analogues and their application in cancer cell imaging†
Abstract
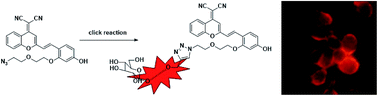
Given the increased glucose uptake in cancer cells than normal cells, near-infrared (NIR) fluorescent glucose analogues have been previously synthesized and applied in cancer cell imaging. However, most NIR dyes usually have one or more charge in their structures, which may cause low cell membrane permeability and hamper their application in cell imaging. Here we report the synthesis and characterization of a series of DCPO-conjugated glucose analogues (N0–N4), which have no charge in their structures and have different lengths of the spacer arm. Experiments in different cancer cell lines showed the uptake of N0–N4 was dependent on the protein levels of GLUT-1. The distance between the dyes and glucose was adjusted by the length of PEG. Of these five glucose analogues, the length of the linker in N2 which contains a diethylene glycol was the most appropriate spacer arm, a longer or shorter linker exhibited reduced cellular uptake efficiency. Moreover, the uptake of DCPO-conjugated glucose analogues could be inhibited by phloretin, a GLUT-1 inhibitor or competitively inhibited by unlabeled D-glucose. Therefore, our study has reported a novel type of NIR-conjugated glucose analogues, whose cell permeability ensured the potential application for cancer cell bioimaging in the NIR region. We also demonstrated, for the first time, that the length of the linker between the dyes and glucose was also an important factor that will affect the delivery efficiency of the glucose analogues to cells.

 Please wait while we load your content...
Please wait while we load your content...