Reactive adsorption of low concentration methyl mercaptan on a Cu-based MOF with controllable size and shape†
Xiang Maab,
Haidi Liua,
Weiman Liab,
Shengpan Pengab and
Yunfa Chen*a
aState Key Laboratory of Multiphase Complex System, Institute of Process Engineering, Chinese Academy of Sciences, Beijing, 100190, China. E-mail: yfchen@ipe.ac.cn; Tel: +86-10-8254-4896
bUniversity of Chinese Academy of Sciences, No. 19A Yuquan Road, Beijing, 100049, China
First published on 3rd October 2016
Abstract
A copper-based metal organic framework (MOF-199) with controllable size and shape was synthesized and used to remove gaseous methyl mercaptan (CH3SH). Characterizations of the synthesized MOF-199 samples before and after desulfurization were carried out by X-ray diffraction (XRD), Fourier transform infrared (FTIR), and X-ray photoelectron spectroscopy (XPS) to observe the adsorption mechanism of CH3SH on MOF-199. The adsorption performance of the synthesized MOF-199 materials and commercialized activated carbon (AC) and Cu loaded AC were evaluated by breakthrough experiments. All three synthesized MOF-199 materials show better performance than commercialized AC. The morphology and texture of MOF-199 materials have great influence on the performance of the adsorption process. The MOF-199 material synthesized by a hydrothermal method exhibited the highest sulfur compound capacity among the synthesized MOF-199 materials (74.7 mg of CH3SH/1 g MOF-199). The color change of MOF-199 during the CH3SH capturing process, indicated a strong interaction between the unsaturated copper sites and –SH group which finally formed CuS and gave rise to obvious damage to the MOF structure.
1. Introduction
Nowadays, odor problems have become a serious concern as environmental and public nuisances with high economic growth and improvement of living standards. Especially, industrial branches such as pharmaceutical factories, insecticide factories and dyestuff plants using thiophene or thiocarbamide are troubled with serious odor problems. Municipal wastewater treatment plants also emit odors and appropriate deodorization methods are used.1 Odor has been recognized as one type of sensory pollution that gives an unpleasant and disgusting feeling and it is often linked directly to the quality of life.Methyl mercaptan (CH3SH) is a kind of typical volatile organosulfur odor in natural gas, petroleum gas, and water gas with a smell like that of rotten or cooked cabbage. CH3SH has a very low olfactory threshold of approximately 0.002 ppm while at high concentrations it is significantly toxic.2 Gas purification techniques such as adsorption, photocatalytic oxidation, biological degradation, thermal decomposition, and catalytic oxidation are emphasized to resolve the challenges aroused by this air pollutant.3–7 However, there are a variety of advantages and disadvantages manifested by these techniques, and they show different degrees of cost effectiveness. For example, biological degradation requires bioreactor with associated aeration system which is expensive. Photocatalytic oxidation needs high maintenance and high system control to avoid residual ozone. Catalytic oxidation needs high ongoing chemical costs with storage and handling of hazardous chemicals.8 Furthermore, all the methods mentioned above except for adsorption could successfully reduce the CH3SH concentration in gas flow from 100 ppm to 10 ppm, these methods, however, could hardly remediate the gas with low CH3SH concentration (<10 ppm) to odorless. Therefore it is of high importance to develop a new technique to make low concentrated CH3SH gas flow free from fetor. In terms of adsorption technique, the adsorption capacity of materials and removal of CH3SH with low concentration remain a challenge because CH3SH molecule is small in size which is not strongly retained on the surface of the adsorbents. That requires adsorption media with highly polar/hydrophilic surface features, which can induce reactive adsorption through hydrogen bonding, complexion and acid–base reactions.9–11 Materials such as activated carbon (AC),12,13 zeolites,14,15 and metal–organic frameworks (MOFs)16 represent the prime candidates for air quality remediation owing to their overall high surface area and surface chemistry.
Metal–organic frameworks are emerging as a class of very important materials offering high levels of porosity with considerable control over pore size and composition. Such properties play a crucial role in functional applications in gas storage or separation, sensing and catalysis.17–21 Their potential versatility, especially in adsorption and enhanced catalytic effects on air-borne pollutants due to high selectivity, chemical and thermal stability in the low and mid temperature range, moderate heat of adsorption, and enhanced mass uptake resulting in advantages over other porous materials such as activated carbon and zeolite.22,23 Recently, adsorptive removal of sulfur-containing compounds by MOFs has received attention because of the increasingly stringent regulations for environmental protection.24–26 MOF-199 (also known as HKUST-1) was one of the first investigated materials to be identified as having a remarkably high capacity for various organosulfur compounds and the structure of MOF-199 was shown in Fig. S1.†27 Petit et al. found that reactive adsorption occurred and resulting in the formation of CuS when MOF-199 was used to adsorb H2S.28 Li et al. carried out adsorption of H2S, CH3CH2SH and CH3SCH3 present in natural gas by MOF-199 and different adsorptive behavior of these sulfur compounds was found through breakthrough experiments.29 Blanco-Brieva et al. found that MOF-199 has a high adsorption capacity of dibenzothiophene due to the interaction between sulfur atom and copper sites of MOF-199.30 However, there are very few reports concerning the morphology effects and adsorbing mechanism of CH3SH on MOF-199. Herein, we propose to use nanoscale MOF-199 with controllable size and shape (large octahedron, denoted as L O; small octahedron, denoted as S O; sphere like, denoted as sphere) as adsorbents for the removal of low concentration CH3SH and all three synthesized materials exhibit better performance than commercialized activated carbon. Moreover, the adsorbing mechanism of CH3SH by MOF-199 was also investigated.
2. Experimental
2.1 Preparation of materials
Cu(NO3)2·3H2O (AR), Cu(CH3COO)2·H2O (AR), ethanol (AR), methanol (AR) were purchased from Xilong Cop. Polyvinyl pyrrolidone (PVP, AR, MW 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), N,N-dimethylformamide (DMF, AR) and AC (wood-based) without further treatment were purchased from Sinopharm. 1,3,5-Benzenetricarboxylic acid (98%) was purchased from Aladdin. The water was made from Millipore Milli-Q water (15 MΩ cm). The gaseous CH3SH was purchased from HuaYuan Gas Chemical industry.
000), N,N-dimethylformamide (DMF, AR) and AC (wood-based) without further treatment were purchased from Sinopharm. 1,3,5-Benzenetricarboxylic acid (98%) was purchased from Aladdin. The water was made from Millipore Milli-Q water (15 MΩ cm). The gaseous CH3SH was purchased from HuaYuan Gas Chemical industry.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). In another flask, 2.077 g of copper nitrate trihydrate was dissolved in 15 ml deionized water. The two solutions were mixed and stirred for 10 min. They were then transferred into Teflon-lined stainless steel autoclave and heated at 80 °C for 16 h. The reaction vessel was then cooled naturally to room temperature. The blue crystals were washed with methanol three times by centrifugation. Then the products were placed in methanol overnight and dried under vacuum at 60 °C for 8 h.
1, v/v). In another flask, 2.077 g of copper nitrate trihydrate was dissolved in 15 ml deionized water. The two solutions were mixed and stirred for 10 min. They were then transferred into Teflon-lined stainless steel autoclave and heated at 80 °C for 16 h. The reaction vessel was then cooled naturally to room temperature. The blue crystals were washed with methanol three times by centrifugation. Then the products were placed in methanol overnight and dried under vacuum at 60 °C for 8 h.2.2 Characterization of materials
The morphology of materials were characterized by field-emission scanning electron microscopy (SEM) via an electron microscope (JEOL 2100F, operating at 15 kV). Analyses were performed on a sample powder previously dried and a sputter coating of a thin layer of gold was performed to avoid specimen charging. The Brunauer–Emmett–Teller (BET) specific surface area and pore size distributions of all catalysts were obtained with N2 adsorption–desorption method on an automatic surface analyzer (AS-1-C TCD, Quantachrome Corp., USA). The pore size distribution and the pore volume of products were calculated by Barrett–Joyner–Halenda (BJH) method. Before measurement, every sample was degassed at 70 °C for 8 h. Powder X-ray diffraction (XRD) patterns of the catalysts were measured on a PANalytical X'Pert PRO system using Cu-Kα radiation in the diffraction angle (2θ) range 5–90° with a step size of 0.03° and scanning rate 0.28 s. Surface species of the as-prepared catalysts were determined by X-ray photoelectron spectroscopy (XPS) using an XLESCALAB 250 Xi electron spectrometer from VG Scientific with a monochromatic Al Kα radiation. Fourier transform infrared (FTIR) spectroscopy was carried out using a Bruker Vertex 70 spectrometer. The spectrum was corrected for the background noise. The experiments were done on the powdered materials 2 wt% mixed with KBr 98 wt%.2.3 Breakthrough testing
The adsorption experiments were carried out in an isothermal plug flow reactor under steady-state conditions at 30 °C by using a water bath. The adsorbents (40–60 mesh, 100 mg) were placed in the U-shaped reactor (diameter 6.0 mm) and the gas flow passing the reactor was 490 ml min−1 (CH3SH: 20 ml min−1, air: 470 ml min−1). The inlet concentration of CH3SH in the mixed gas was about 5 ppm. A detailed adsorption system is shown in Fig. S2.† The value of “breakthrough” in this study was 4% of the feed concentration which was about 0.2 ppm. The time at which the concentration of CH3SH in the effluent surpassed the breakthrough concentration is designated as the “breakthrough time”. The concentration of CH3SH in the mixed gas was analyzed by a CH3SH detector with detection line of 0.01 ppm (Yuan technology Co., Ltd, SKY 2000 series). We had conducted blind measurement with 100 mg quartz sand which is a typical non-porous material. The dead time is less than 1 s according to our experiment which is negligible comparing with our long adsorbing time. Before we recorded the adsorbing time, we filled the whole system with 5 ppm CH3SH (CH3SH: 20 ml min−1, air: 470 ml min−1), the dead volume was estimated to be about 10 cm3 which is negligible comparing with the volume of the whole adsorption system.| Dead volume = πR2l = 3.14 × 0.3 × 0.3 × 35 = 9.89 cm3 (l represents the length of U-shaped reactor) |
3. Results and discussion
3.1 Characterizations of materials
The chemical composition and crystal morphology of the synthesized MOF-199 materials are expected to influence their adsorptive properties of CH3SH. Since our goal was their potential application as adsorbents of CH3SH, the morphology is considered as one important factor. The morphology of three synthesized MOF-199 materials were examined by SEM. Fig. 1a exhibits large octahedral structure prepared by conventional hydrothermal method with size of about 4 μm. Other than the conventional recipe of large octahedral structure, PVP was employed as the capping additive to alter the coordination equilibrium at the crystal surface during the growth process in the synthesis. Fig. 1b shows the small octahedral morphology with size of about 300 nm, which is formed through adding 1,3,5-benzenetricarboxylic acid solution drop by drop into the copper nitrate solution. Fig. 1c illustrates the sphere like morphology of MOF-199 with distinguishable meso- and macropores which are generated from the spaces among the interconnected sphere crystals. Comparing with L O and S O, more surface defects exist on sphere like MOF-199. The SEM image of commercialized AC and Cu loaded AC is illustrated in Fig. S3.†The crystallographic structures of the three synthesized MOF-199 materials were determined by XRD measurements. Fig. 2 shows the XRD patterns of L O, S O, sphere and the simulation pattern of HKUST-1 which confirms that these crystals have the same structure as HKUST-1 previously reported.32,33 Both samples showed good crystallinity and the XRD patterns of the three synthesized materials are in good agreement with the simulation pattern of HKUST-1 with the formula of Cu3(C9H3O6)2(H2O)3 which was first reported by Chui et al.34 However some deviation is observed in the relative intensities because of variations in the degree of hydration.35 The large octahedral structure prepared by conventional hydrothermal method has the best crystallinity among the synthesized MOF-199 materials because hydrothermal conditions are beneficial to the nucleation and crystal growth of MOF-199 material. On the contrary, the crystallinity of sphere like MOF-199 shown in Fig. 2 is relative weaker due to the room temperature synthesis of MOF material without relative higher temperature and pressure treatment.36 The crystallinity of S O MOF-199 is better than sphere due to the introduction of PVP since PVP acts as a structure-directing agent which could facilitate the crystallisation of MOF domains through the interaction between pyrrolidone rings (C–O) and metal ions.37 The XRD patterns of Cu loaded AC with different loading amount is shown in Fig. S4.†
The porosity is considered as of paramount importance to the adsorption of hazardous pollutants. A difference among synthesized MOF-199 materials is revealed by specific surface area analysis and pore measurement. Fig. 3 displays the N2 adsorption–desorption isotherms and the pore size distribution of the as-prepared adsorbents. The textural parameters of all samples are summarized in Table 1.
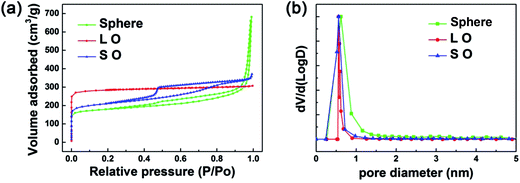 | ||
| Fig. 3 (a) N2 adsorption–desorption and (b) pore size distributions of the synthesized MOF-199 materials. | ||
| Sample | BET surface area (m2 g−1) | Pore volume (cm3 g−1) |
|---|---|---|
| L O | 903.9 | 0.477 |
| S O | 684.5 | 0.576 |
| Sphere | 580.7 | 1.060 |
| AC | 729.8 | 0.539 |
The surface area and pore volume of L O, S O, sphere like MOF-199 were 903.9 m2 g−1 and 0.477 cm3 g−1, 684.5 m2 g−1 and 0.576 cm3 g−1, 580.7 m2 g−1 and 1.06 cm3 g−1 respectively. The comparison of BET surface area between the synthesized MOF-199 in our work and previously work are summarized in Table S1.† The difference of BET surface area may result from different activation temperature, reaction temperature, time, solvent reagent, concentration and nature of the precursors used. The L O MOF-199 possessing highest surface area and smallest pore volume among the synthesized MOF-199 materials and there is no mesoporous structure formed within L O MOF-199. It is noted that the BET surface area of S O and sphere like MOF-199 are lower than that of L O, which is mainly ascribed to partial inclusion of PVP and lower crystallinity, respectively.38 The average pore diameter of the three synthesized MOF-199 materials is about 0.6 nm which is in accordance with the MOF-199 structure. The high surface area in synthesized MOF-199 materials could provide more active sites and highly porous structure would be more favorable for the adsorption and diffusion of reactant molecules. The N2 adsorption–desorption isotherms and the pore size distribution of commercialized AC is illustrated in Fig. S5.†
3.2 Adsorption of gaseous CH3SH
Breakthrough curves for CH3SH adsorption in three synthesized MOF-199 samples and commercialized AC were recorded. Furthermore, the breakthrough curved of Cu loaded AC with different loading amount was shown in Fig. S6.† The best performance sample of Cu loaded AC was labelled as Cu-0.7. The preparation method of Cu loaded AC was illustrated in the ESI.† Selected plots of breakthrough curves and estimated dynamic adsorption capacities for CH3SH are presented in Fig. 4. It is indicated that the commercialized AC performed worst in the adsorption process which is ascribed to the lack of active sites on the surface although the BET surface area of AC is relative high. However, with the modification with Cu, the adsorption property of AC was obviously enhanced because of formation of Cu, CuO and Cu2O species which is active for the elimination of CH3SH during adsorption process.39 The L O MOF-199 sample had the highest adsorption capacity which is almost 10 times higher than that of AC. The L O MOF-199 material could last about 26 h till the outlet concentration reached the “breakthrough” value. The largest BET surface area of L O which facilitated the adsorption of CH3SH molecular contributed to the good performance during the adsorption process. Interestingly, the sphere MOF-199 which possesses the smallest BET surface area also performed well. We considered that meso- and macropores which are generated from spaces among the interconnected sphere crystals are crucial in the adsorption process acting as bypass which facilitate the transportation and adsorption of CH3SH when the small MOF-199 crystals got adsorbed with CH3SH.40,41 While S O had the lowest sulphur capacity among three synthesized MOF-199 materials because of partial inclusion of PVP which occupied the active sites of S O MOF-199 resulting in bad performance and illustration of remaining PVP was shown in Fig. S7.† The comparison of sulphur compound capacity between L O MOF-199 and previously reported work is summarized in Table S2.† The sulphur compound capacity of L O MOF-199 is higher than most other sulphur compound such as SO2, C2H5SH, CH3SCH3 and dibenzothiophene adsorbed on HKUST-1. It is a little lower than H2S adsorbed on HKUST-1 combined with GO because the introduction of modified GO can form new microporosity as a result of linkages between the sulfonic acids and amine groups of modified GO and copper sites in MOF-199. The adsorption mechanism of CH3SH leading to high sulphur compound capacity on MOF-199 was described below.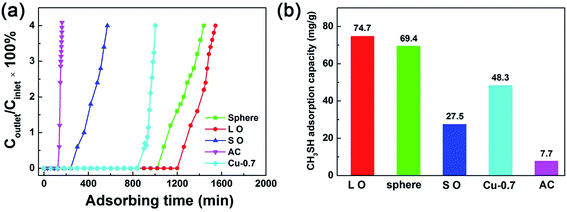 | ||
| Fig. 4 (a) CH3SH breakthrough curves and (b) adsorption capacity for as-synthesized MOF-199 materials, commercialized AC and Cu-0.7. | ||
3.3 Mechanism of adsorption
To determine the desulfurization mechanism, we characterized the exhausted samples through FTIR, XRD, and XPS measurements and compared results against fresh samples using the best performance L O MOF-199 material. Fig. 5a presents the IR spectra of fresh and exhausted L O MOF-199. Characteristic bands for MOF-199 representing the stretching vibrations of the functional groups are clearly visible.42 For example, bands located at 1108.5 cm−1 and 941.8 cm−1 resulted from C–O–Cu stretching. The symmetric stretching vibrations of the carboxylate group were observed at 1643.0 cm−1, whereas the asymmetric modes were observed at 1452.7 cm−1 and 1372.0 cm−1. In comparison to the fresh L O MOF-199, noticeable changes were observed after absorbing CH3SH. There new bands appeared at 1708.1 cm−1, 1275.4 cm−1 and 683.5 cm−1. The first two peaks and the latter peak are ascribed to carboxylate protonation and Cu–S vibration, respectively.43 These results indicate that chemical adsorption occurred most likely at the copper site in the MOFs. Fig. 5b displays the XRD patterns of fresh and exhausted L O MOF-199. The intensity of peaks in the exhausted spectrum is noticeably lower, with only the main peaks remaining. The disappearance or great reduction of XRD characteristic peaks and the color change indicate that, because of a strong chemical reaction, the molecular structure of MOF-199 severely collapsed after adsorbing CH3SH. | ||
| Fig. 5 (a) FTIR spectra for L O MOF-199 before and after desulfurization (b) XRD patterns of L O MOF-199 before and after desulfurization. | ||
To reveal the chemisorptions mechanism and the state of constituent elements, XPS of the fresh and exhausted L O MOF-199 was performed. Assignments of separate peaks in the XPS Cu 2p3/2 and S 2p spectra are listed in Table S3.† The Cu 2p3/2 spectrum of the fresh L O MOF-199 is in accordance with that in the literature.44 While the Cu 2p3/2 spectrum of exhausted sample was quite different from the fresh one with the appearance of CuS located at 933.2 eV.45 The S 2p spectrum of the exhausted sample was fitted into two pairs of 2p3/2 and 2p1/2 doublets. The first pair located at 162 eV and 162.6 eV are attributed to surface tangling sulfur anions, and the second pair located at 163.4 eV and 164.3 eV are assigned to the lattice sulfur of CuS in the surface region.46,47 The peak located at 168.3 eV is assigned to the adsorbed methyl thiolate.48 Apparently, CuS was formed during adsorption, coinciding with the color change of L O MOF-199 before and after the adsorption process (Fig. 6).
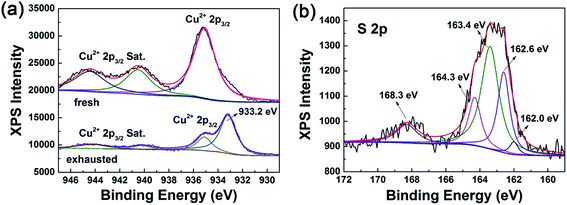 | ||
| Fig. 6 (a) XPS spectra for Cu 2p3/2 of fresh and exhausted L O MOF-199 (b) XPS spectra for S 2p of exhausted L O MOF-199. | ||
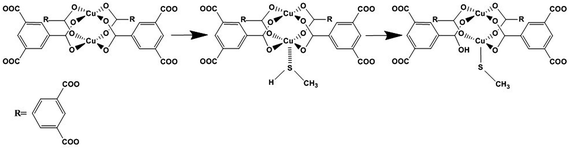
XPS findings are in good accordance with previous results from IR spectroscopy and XRD measurements. The synthesized MOF-199 materials have open sites of copper that exhibit Lewis acidity and, thus, the ability to adsorb compounds with long electron pairs, such as sulfur compounds. The small size of CH3SH molecule and the small hydrogen atom bound to sulfur at the end make it easy to access the open copper site by coordination. Upon extended exposure to CH3SH, the strong affinity between copper and sulfur may break the original Cu–O bond, form a new bond (O–Cu–S–CH3) and release carboxylic groups. A fraction of –SCH3 can further break down, resulting in the formation of CuS. Nevertheless, because the binding energy of S in O–Cu–S–CH3 might be very close to that of CuS,49 XPS results could not show the difference between them. The reactive adsorption of CH3SH molecules create a different chemical environment for Cu and eventually result in the color change of MOF-199 material and the adsorption process is shown in Scheme 1. The adsorption process is similar to the adsorption of H2S on MOF-199 which reactive adsorption occurred and resulted in structure collapse as well as obvious colour change of MOF material.
4. Conclusions
MOF-199 materials with controllable size and shape (L O, S O, sphere like) were successfully synthesized to adsorb gaseous CH3SH. All three synthesized MOF-199 materials exhibit better performance than commercialized AC and Cu loaded AC by breakthrough experiments due to the unsaturated copper sites. It was found that the morphology and texture of MOF-199 materials have great impact on the adsorbing property. The existence meso- and macropores can facilitate the transportation and adsorption of CH3SH molecules resulting in relative high capacity of CH3SH. On the contrary, the partial inclusion of PVP which occupied the active sites of MOF-199 resulted in bad performance of CH3SH adsorption. Reactive adsorption was found because of the strong interaction between the unsaturated copper sites in MOF-199 and –SH group of CH3SH which resulted in the formation of CuS and serious molecular structure collapse. The L O MOF-199 sample synthesized by hydrothermal method which exhibited the highest capacity of CH3SH due to their highest BET surface area makes potential application for CH3SH removal.Acknowledgements
The authors are grateful to the Strategic Project of Science and Technology of Chinese Academy of Science (No. XDB05050400) and the National Science and Technology support (No. 2014BAC21B00).References
- P. Lewkowska, B. Ciešlik, T. Dymerski and P. Konieczka, Environ. Res., 2016, 151, 573–585 CrossRef CAS PubMed.
- D. J. Kim and J. E. Yie, J. Colloid Interface Sci., 2005, 283, 311–315 CrossRef CAS PubMed.
- C. H. Tsai, W. J. Lee, C. Y. Chen and W. T. Liao, Ind. Eng. Chem. Res., 2001, 40, 2384–2395 CrossRef CAS.
- R. Lebrero, A. C. Gondim, R. Pérez, P. A. García-Encina and R. Muñoz, Water Res., 2014, 49, 339–350 CrossRef CAS PubMed.
- S. Z. Zhao, H. H. Yi, X. L. Tang, F. Y. Gao and B. W. Zhang, J. Cleaner Prod., 2015, 87, 856–861 CrossRef CAS.
- X. Z. Li, M. F. Hou, F. B. Li and H. Chua, Ind. Eng. Chem. Res., 2006, 45, 487–494 CrossRef CAS.
- T. X. Liu, X. Z. Li and F. B. Li, Environ. Sci. Technol., 2008, 42, 4540–4545 CrossRef CAS PubMed.
- A. Shammay, E. C. Sivert, N. Le-Minh, R. L. Fernandez, I. Evanson and R. M. Stuetz, J. Environ. Chem. Eng., 2016, 4, 3866–3881 CrossRef CAS.
- S. Bashkova, A. Bagreev and T. J. Bandosz, Catal. Today, 2005, 99, 323–328 CrossRef CAS.
- S. W. Lee, W. M. A. W. Daud and M. G. Lee, J. Ind. Eng. Chem., 2010, 16, 973–977 CrossRef CAS.
- H. Tamai, H. Nagoya and T. Shiono, J. Colloid Interface Sci., 2006, 300, 814–817 CrossRef CAS PubMed.
- S. Bashkova, A. Bagreev and T. J. Bandosz, Environ. Sci. Technol., 2002, 36, 2777–2782 CrossRef CAS PubMed.
- S. Bashkova, A. Bagreev and T. J. Bandosz, Ind. Eng. Chem. Res., 2002, 41, 4346–4352 CrossRef CAS.
- C. Cammarano, E. Huguet, R. Cadours, C. Lerop, B. Coq and V. Hulea, Appl. Catal., B, 2014, 156, 128–133 CrossRef.
- A. Ryzhikov, V. Hulea, D. Tichit, C. Leroi, D. Anglerot, B. Coq and P. Trens, Appl. Catal., A, 2011, 397, 218–224 CrossRef CAS.
- X. W. Liu, T. J. Sun, J. L. Hu and S. D. Wang, J. Mater. Chem. A, 2016, 4, 3584–3616 CAS.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. V. Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- Y. J. Cui, B. Li, H. J. He, W. Zhou, B. L. Chen and G. D. Qian, Acc. Chem. Res., 2016, 49, 483–493 CrossRef CAS PubMed.
- D. Wisser, F. M. Wisser, S. Raschke, N. Klein, M. Leistner, J. Grothe, E. Brunner and S. Kaskel, Angew. Chem., Int. Ed., 2015, 54, 1–5 CrossRef PubMed.
- M. Kurmoo, Chem. Soc. Rev., 2009, 38, 1353–1379 RSC.
- C. Y. Huang, M. Song, Z. Y. Gu, H. F. Wang and X. P. Yan, Environ. Sci. Technol., 2011, 45, 4490–4496 CrossRef CAS PubMed.
- P. Sliva, S. M. F. Vilela, J. P. C. Tomé and F. A. A. Paz, Chem. Soc. Rev., 2015, 44, 6774–6803 RSC.
- P. Kumar, K. H. Kim, E. E. Kwon and J. E. Szulejko, J. Mater. Chem. A, 2016, 4, 345–361 CAS.
- E. Barea, C. Montoro and J. A. R. Navarro, Chem. Soc. Rev., 2014, 43, 5419–5430 RSC.
- A. M. Ebrahim, J. Jagiello and T. J. Bandosz, J. Mater. Chem. A, 2015, 3, 8194–8204 CAS.
- J. Song, Z. Luo, D. K. Britt, H. Furukawa, O. M. Yaghi, K. L. Hardcastle and C. L. Hill, J. Am. Chem. Soc., 2011, 133, 16839–16846 CrossRef CAS PubMed.
- D. Britt, D. Tranchemontagne and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11623–11627 CrossRef CAS PubMed.
- C. Petit, B. Levasseur, B. Mendoza and T. J. Bandosz, Microporous Mesoporous Mater., 2012, 154, 107–112 CrossRef CAS.
- Y. Li, L. J. Wang, H. L. Fan, J. Shangguan, H. Wang and J. Mi, Energy Fuels, 2015, 29, 298–304 CrossRef CAS.
- G. Blanco-Brieva, J. M. Campos-Martin, S. M. Al-Azhrani and J. L. G. Fierro, Fuel, 2011, 90, 190–197 CrossRef CAS.
- J. L. C. Rowsell and O. M. Yaghi, J. Am. Chem. Soc., 2006, 128, 1304–1315 CrossRef CAS PubMed.
- S. Y. Zhang, H. Liu, C. C. Sun, P. F. Liu, L. C. Li, Z. H. Yang, X. Feng, F. W. Huo and X. H. Li, J. Mater. Chem. A, 2015, 3, 5294–5298 CAS.
- J. L. Zhuang, D. Ceglarek, S. Pethuraj and A. Terfort, Adv. Funct. Mater., 2011, 21, 1442–1447 CrossRef CAS.
- S. S.-Y. Chui, S. M.-F. Lo, J. P. H. Charmant, A. G. Orpen and L. D. Williams, Science, 1999, 283, 1148–1150 CrossRef CAS PubMed.
- K. Schlichte, T. Kratzke and S. Kaskel, Microporous Mesoporous Mater., 2004, 73, 81–88 CrossRef CAS.
- S. L. Qiu and G. S. Zhu, Coord. Chem. Rev., 2009, 253, 2891–2911 CrossRef CAS.
- Z. C. Zhang, Y. F. Chen, X. B. Xu, J. C. Zhang, G. L. Xiang, W. He and X. Wang, Angew. Chem., Int. Ed., 2014, 53, 429–433 CrossRef CAS PubMed.
- G. W. Zhan and H. C. Zeng, Chem. Commun., 2016, 52, 8432–8435 RSC.
- D. J. Kim and J. E. Yie, J. Colloid Interface Sci., 2005, 283, 311–315 CrossRef CAS PubMed.
- A. J. Amali, J. K. Sun and Q. Xu, Chem. Commun., 2014, 50, 1519–1522 RSC.
- W. X. Tang, X. F. Wu, S. D. Li, X. Shan, G. Liu and Y. F. Chen, Appl. Catal., B, 2015, 162, 110–121 CrossRef CAS.
- R. K. Bhakta, J. L. Herberg, B. Jacobs, A. Highley, R. Behrens, N. W. Ockwig, J. A. Greathouse and M. D. Allendorf, J. Am. Chem. Soc., 2009, 131, 13198–13199 CrossRef CAS PubMed.
- B. Levasseur, C. Petit and T. J. Bandosz, ACS Appl. Mater. Interfaces, 2010, 12, 3606–3613 Search PubMed.
- A. S. Duke, E. A. Dolgopolova, R. P. Galhenage, S. C. Ammal, A. Heyden, M. D. Smith, D. A. Chen and N. B. Shustova, J. Phys. Chem. C, 2015, 119, 27457–27466 CAS.
- C. Y. Wu, S. H. Yu, S. F. Chen, G. N. Liu and B. H. Liu, J. Mater. Chem., 2006, 16, 3326–3331 RSC.
- R. Cai, J. Chen, J. X. Zhu, C. Xu, W. Y. Zhang, C. M. Zhang, W. H. Shi, H. T. Tan, D. Yang, H. H. Hng, T. Mariana and Q. Y. Yan, J. Phys. Chem. C, 2012, 116, 12468–12474 CAS.
- S. L. Xiong and H. C. Zeng, Angew. Chem., Int. Ed., 2012, 51, 949–952 CrossRef CAS PubMed.
- S. Z. Zhao, H. H. Yi, X. H. Tang, F. Y. Gao, B. W. Zhang, Z. X. Wang and Y. R. Zuo, J. Cleaner Prod., 2015, 87, 856–861 CrossRef CAS.
- V. Srinivasan, E. I. Stiefei, A. Elsberry and R. A. Walton, J. Am. Chem. Soc., 1979, 101, 2611–2614 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18593b |
| This journal is © The Royal Society of Chemistry 2016 |