Highly symmetric spongy porous poly(ether sulfone) membranes with selective open-cells for vanadium flow battery application†
Dongju Chen*a,
Dandan Lia and
Xianfeng Li*b
aSchool of Chemistry and Chemical Engineering, Liaoning Normal University, Huanghe Road 850, Dalian 116029, China. E-mail: dongju.chen@yahoo.com
bDivision of Energy Storage, Dalian Institute of Chemical Physics, Chinese Academy of Science, Zhongshan Road 457, Dalian 116023, China. E-mail: lixianfeng@dicp.ac.cn
First published on 7th September 2016
Abstract
Poly(ether sulfone)/sulfonated poly(ether ether ketone) (PES/SPEEK) symmetric spongy porous membranes are prepared for vanadium flow battery (VFB) application. The membrane morphology can be easily controlled by changing the hydrophilic SPEEK content in the cast solution. The performance of the spongy porous PES membranes with different morphologies was studied in detail. The results indicate that the closed spongy pores are more connected and the ultra-thin pore walls are thinner after introducing SPEEK into the cast solution, resulting in lower columbic efficiency (CE) along with higher voltage efficiency (VE). The optimized VFB performance of the PES porous membrane illustrated a CE of 92.98% and VE of 83.60% and the performance remained stable after running for more than 200 cycles. In addition, the prepared PES porous membrane showed an excellent capacity retention capability as well, showing good potential usage in VFBs for long term operation.
Introduction
The serious issues of energy shortage and environmental pollution are promoting the development of renewable energies like solar and wind power. However, these renewable energy sources are random and intermittent, which make them quite challenging to use and to connect with the grid. Therefore, the development of electrical energy storage becomes a valid strategy for improving the reliability of the grids.1,2 Among different types of techniques, a vanadium flow battery (VFB), which perfectly combines the attractive features of high durability, high safety and high efficiency, is regarded as an ideal choice for large-scale power conversion systems.3,4 Normally a VFB comprises two external reservoirs, which are filled with electrolytes or different valence vanadium ions, two pumps and a battery cell. The charge–discharge process was realized via the transformation of the oxidation state of vanadium ions. To achieve this purpose, a membrane is used to isolate the electrolytes and to allow protons transportation to complete the current circuit.5 Accordingly, to develop a chemically stable, low cost and high quality membrane is of high importance.6Perflurosulfonic polymer based membranes (for example, Nafion series) have so far been widely used in a VFB,7,8 because of their high proton conductivity and high oxidation stability. However, the low ion selectivity and too high cost of these membranes hindered the commercialization of a VFB.9 Recent studies of non-perfluoronated polymers such as quaternary or sulfonated ammoniated aromatic polymers have been studied widely for VFB application due to their characteristics like tunable ion selectivity and low cost.10,11 However, studies have also shown that these membranes are likely to be significantly limited by membrane degradation during charge/discharge process.12–14 Therefore, considerable efforts have been focused on developing substitute membranes with low cost, high performance and high chemical stability.
Porous nanofiltration membranes, which could separate vanadium ions from protons via pore size exclusion, have attracted keen interest because they overcame the issues of poor chemical stability caused by the ion exchange groups.15 Porous membranes such as poly(vinylidene fluoride) (PVDF), poly(ether sulfone) and polyacrylonitrile (PAN) were successfully fabricated through traditional phase inversion method and have been studied in a VFB.15–17 Normally, these membranes own an asymmetric structure, which has a selective skin layer and a support layer.18 It is well known that the pore size and pore size distribution are crucial to determine the ion selectivity of a membrane. For VFB application, smaller pores in skin layer normally render a higher ion selectivity; however, they are prone to induce higher ion transport resistance.19,20 In response to this considerable challenge, novel advances in membrane modification and morphology control have been carried out, e.g. polymer blending, interfacial polymerization and hydrophilic treatment.5,21 Recently, an efficient method based on the solvent-responsive layer-by-layer assembly (SR-LBL) was used to fabricate porous nanofiltration membrane with well-defined ions transport channels.22 This approach perfectly solved the conflict between ion conductivity and ion selectivity. Further improvement in this conflict can be realized by designing weak alkaline membrane with highly symmetric spongy structure via the vapor induced phase inversion method.9 However, few studies followed using other polymer resins due to the fact that membranes formed through vapor induced phase inversion method show closed cells, which will result in too high ion transport resistance.
In this paper, poly(ether sulfone) (PES) was selected as the matrix for spongy porous membrane fabrication owning to its excellent chemical and mechanical stability.23 Negatively charged SPEEK was introduced on pore walls to tune the membranes morphology and conductivity. The morphology of the membranes was tuned via changing the content of positively charged SPEEK in solutions. Their ion conductivity, ion selectivity together with VFB single cell performance was investigated in detail.
Experimental
Materials
Poly(ethersulfone) (PES) was kindly supplied by Changchun Jilin University Special Plastic Engineering Research. SPEEK was fabricated as reported by direct sulfonation of PEEK, the sulfonation reaction was carried out by sulfonating with sulfuric acid at 70 °C for 2 hours.24 The degree of sulfonation, which was detected by 1H NMR (Fig. S1 in the ESI†), is 0.78.Membrane preparation
The sponge porous membranes were prepared via water vapor induced phase inversion (WVIP) method. The solution was prepared by dissolving SPEEK and PES blend polymers in DMAc, the membrane morphology was further tunned by changing the weight ratio of PES to SPEEK. The solution was firstly cast on a glass plate, then, the casted plate was quickly switched to a constant climate chamber for about 10 min. The temperature and humidity of the chamber were kept at 50 °C and 100% respectively (referred as M-a-b, where a and b are the total polymer concentration and SPEEK content ratio in total polymer, respectively). Finally, the membrane was peeled off and treated by water to remove DMAc. The thickness of the prepared membrane is 85 ± 5 μm.Membrane characterization
where VB is the volume solution of rightcell, CB(t) is VO2+ concentration of the solution in the right cell as a function of time t, L and A are the thickness and effective area of a membrane, respectively. P is the permeability of VO2+, CA is VO2+ concentration of the solution in left cell.
| R = (R1 − R2) × S |
Results and discussion
SEM was carried out to investigate the cross section and surface morphology of PES porous membranes. Normally membranes that were prepared by traditional phase separation method have an asymmetric structure, which possesses a porous support layer and a thin selective skin layer.19 In this paper, the membrane, which was prepared by WVIP method showed a symmetric spongy morphology.26 As shown in Fig. 1a–d, the cross sections of PES porous membranes prepared from different SPEEK content (increased from 12% to 15%) through WVIP method all exhibit spongy-like pores, which are similar to the structure of chloromethylated poly sulfone (CMPSF).27 The membrane showed a typical spongy-like structure composed of closed pores (Fig. 1b′ and d′). With SPEEK content increasing, the pore size increases. In addition, with the increase of the SPEEK content, no distinguished spherulitic structures are observed on the surface in Fig. 1e–h (facing the water vapor side). It has been well established that the membrane morphology is closely related to the phase separation kinetics as well as thermodynamic equilibrium.28,29 The kinetic aspect describes the composition and the change of the polymer concentration during the membrane forming process. While for thermodynamic aspect, which is based on the Flory Huggins theory, reveals the stability border of the dope solution and the type of phase separation (spontaneous demixing and delay demixing).30 Various researches have been demonstrated that the difference between traditional phase inversion method (asymmetric morphology) and WVIP method (symmetric morphology) is not resulted from the thermodynamic properties, but from the kinetic properties.31 The asymmetric morphology, resulted from the kinetic properties is closely related to concentration distribution and activity gradients of non-solvent, the miscibility between solvent and polymer. During the phase inversion process, the quick exchange rate between solvent and non-solvent at the interface will lead to a fixed chemical composition in a very short time and further to a phase separation. In addition, a dense skin layer will be formed at the film surface, which can further decrease the exchange rate between the solvent and non-solvent, which favoured the formation of asymmetric morphology. In the case of WVIP method, the even concentration distribution of solvent, non-solvent and polymer through the polymer solution film, induces polymer solution precipitating simultaneously over film cross section, further forming a symmetric membrane morphology.TEM was carried out on M-35-12 and M-35-13 membranes to further investigate the influence of SPEEK on the morphology of prepared membranes. The results are shown in Fig. 2. The white zones correspond to the pores in membranes. Similarly, the pores become larger with increasing SPEEK content. The pores switched from closed (Fig. 2a) to open (Fig. 2d), connecting with each other and forming more continues pores when the weight content of increased from 12% to 13%. It should be noted that with increasing amount of SPEEK in the cast solution, the ultra-thin pore walls may become thinner (Fig. 2b and e), which will be beneficial to increasing the proton transport rate through a membrane. The structure of the membrane was determined by the driving force and diffusion rate of the solvent and nonsolvent. The SPEEK would favor water ingression since SPEEK was a hydrophilic polymer. In addition, the SPEEK was a charged polyelectrolyte, where the electrostatic repulsion could impede the bundling of polymer, resulting in the interconnected pore. As a result, the addition of SPEEK could increase pore size of the membranes. The dark spots in Fig. 2c and f are the clusters, which are formed by the interactions between Ag+ and negative charged sulfonic acid groups, indicating the distribution of sulfonic acid groups on the pore walls.
 | ||
| Fig. 2 TEM images of PES porous membranes stained with Ag+ under increased magnification from (a)–(c) (M-35-12) and (d)–(f) (M-35-13). | ||
In a VFB, the vanadium ions permeability through a membrane will bring about capacity fade and self-discharge. Therefore, the vanadium ions permeability will largely affect the VFB performance. Fig. 3a shows a liner curve of time vs. the VO2+ concentration of membranes prepared from different SPEEK contents. The slope of the line reflects the permeability of VO2+ through PES porous membrane, indicating that the permeability of VO2+ improved with the increasing SPEEK content. This was due to the fact that larger pores (Fig. 2) were formed with increasing SPEEK amount. In addition, the interaction between VO2+ and sulfonic groups of SPEEK also favoured the permeability of VO2+.
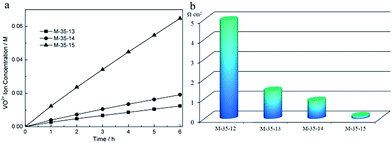 | ||
| Fig. 3 (a) VO2+ concentration versus diffusion time of PES porous membranes; (b) area resistance of PES porous membranes. | ||
The area resistance can effectively reflect the ion conductivity of a membrane and finally determine the internal resistance of a battery. As shown in Fig. 3b, the area resistance decreases from 4.96 Ω cm2 to 0.13 Ω cm2 sharply from M-35-12 to M-35-15. The addition of SPEEK was benefit to the transportation of protons, which is due to the interaction between sulfonic groups and protons. Meanwhile, the increased content of sulfonic groups would induce membranes with larger and more connected pores, favouring the proton transportation and finally decreasing the area resistance. These results are in according to morphology and vanadium permeability results.
Fig. 4a demonstrated the single cell performance of VFBs, which are assembled with the prepared membranes. The results showed that when SPEEK content is kept at 12%, the VFB assembled with M-35-12 membrane exhibits too high ohmic overpotential and cannot be charged due to the too high resistance of the membranes. Then increasing SPEEK content from 13% to 15%, the columbic efficiency (CE) of the porous membranes, which reflects the ions selectivity of a membrane, decreased from 91.12% to 61.43%, while the voltage efficiency (VE), increased from 86.10% to 90.39%. Accordingly, the energy efficiency, which is the product from VE and CE, decreased continuously from 78.31% to 55.52%. It can be seen that a slightly increased SPEEK content will result in a significant difference of VFB performance. With the increasing SPEEK content, the pore walls were fully negatively charged and prone to transfer from closed to open and the pores become larger and more connected, which is beneficial for transporting of protons, and further resulting in a higher VE. However, the multiple pore walls, which function as multi-layer barriers to separate protons from vanadium ions by their difference in radius size, turns to more interconnecting through the open pores, leading to the high permeability of vanadium ions, as a result, a lower CE was obtained. Fig. 4b showed the performance of VFB single cell assembled with membranes, which are prepared from solutions with different concentration. The polymer concentration was varied from 25 wt% to 37 wt%. The CE increases and VE decreases with increasing polymer concentration, due to smaller and more disconnected pores of membranes, which is induced by high viscosity of the solutions. The VFB performance of M-35-13 operating at different current densities (40–160 mA cm−2) was also measured (Fig. 4c). As expected, the CE increases, while VE decreases with the current density increasing from 40 mA cm−2 to 160 mA cm−2. This tendency is due to the shorter charge/discharge time and the higher overpotential at higher current density.
Normally, a membrane fabricated via the WVIP method composed of closed pores,28 which were inimical to the transportation of protons and further resulted in a high area resistance. Accordingly, it is desirable to prepare symmetric membrane morphology with selective open-closed pores through adding different SPEEK content in the cast solution to better understand the structure property relationships (Fig. 5), which will lead to improved and tailored symmetric membrane for vanadium flow battery.
The capacity decay of a VFB that employ an ion exchange membrane such as Nafion 115 is a technical obstacle haunting the commercialization of VFB technology.32 The capacity decay occurs primarily due to different transfer rates of vanadium ions with different valences across the membrane during operation.33 Continuous capacity decay will induce undesired side reactions, and further reducing the system capacity, as a result, a more frequent maintenance will be required and operation cost of a VFB system will increase as well.34,35 Therefore, high capacity retention is highly important for practical application of VFB. In this work, the PES porous membrane exhibits excellent ability to maintain high capacity retention over extend cycling as shown in Fig. 6a. In addition, no remarkable capacity fading was found on the battery after continuous running for 20, 80, 140, 200 cycles (Fig. 6b), which further illustrates that the prepared porous membrane owns high capacity retention under VFB operation medium. Therefore, the use of PES porous membrane in VFB can potentially decrease the maintenance cost and further improve overall system efficiency.
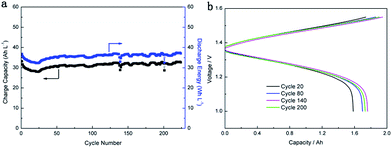 | ||
| Fig. 6 (a) Charge capacity and discharge energy over cycling of M-35-13 PES porous membrane and (b) charge and discharge curves in VFB operation (80 mA cm−2) with different cycles. | ||
The cycle life test was carried out on a VFB assembled with M-35-13 at a current density of 80 mA cm−2. As shown in Fig. 7a, the CE was kept at around 91% while, the VE remained about 86% over 200 cycles, indicating the good chemical stability of prepared PES porous membrane. FTIR was employed to confirm the chemical stability of the prepared PES porous membrane before and after cycle test. The FTIR results were displayed in Fig. 7b. The full FTIR spectra did not show obvious difference between the initial membrane and membrane after cycle test, indicating again the good chemical stability of prepared PES porous membrane during cycle test. To further confirm the membrane stability, SEM was performed on the surface facing the positive side and cross section and magnification cross section (Fig. S2 in the ESI†) of the membrane after cycling test (Fig. 7c), the membrane surface remains smooth after lifetime test, and the cross section (Fig. 7c) exhibits no significant changes throughout the membrane as well, indicating the excellent chemical stability of PES porous membrane.
Conclusions
In summary, PES porous membranes with highly symmetric spongy structure were prepared via WVPI method. This symmetric spongy structure with well controlled morphology was realized by the adding hydrophilic SPEEK in the cast solution. The morphology of the prepared membranes was detected by SEM and TEM. With the increasing SPEEK content in the cast solution, the closed pores become more open and interconnected along with thinner ultra-thin pore walls. As a result, the performance of a VFB assembled with a PES porous membrane with optimized structure showed CE of 91.12% and VE of 86.10% at a current density of 80 mA cm−2. And the VFB cell showed a stable performance after running more than 200 cycles. Moreover, the optimized porous membrane showed an excellent ability to maintain high capacity retention, which can realize the stability and simple VFB operation during long term cycling. Therefore, PES porous membranes with well controlled morphologies show very good potential usage in VFB.Acknowledgements
Dr X. F. Li greatly acknowledges the financial support from China Natural Science Foundation (Grant No. 21476224).Notes and references
- Z. Yang, J. Zhang, M. C. Kintner-Meyer, X. Lu, D. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 3577–3613 CrossRef CAS PubMed.
- B. Dunn, H. Kamath and J.-M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- C. Ding, H. Zhang, X. Li, T. Liu and F. Xing, J. Phys. Chem. Lett., 2013, 4, 1281–1294 CrossRef CAS PubMed.
- M. Skyllas-Kazacos, M. H. Chakrabarti, S. A. Hajimolana, F. S. Mjalli and M. Saleem, J. Electrochem. Soc., 2011, 158, R55–R79 CrossRef CAS.
- D. Zhang, X. Yan, G. He, L. Zhang, X. Liu, F. Zhang, M. Hu, Y. Dai and S. Peng, J. Mater. Chem. A, 2015, 3, 16948–16952 CAS.
- Z. Yuan, X. Zhu, M. Li, W. Lu, X. Li and H. Zhang, Angew. Chem., Int. Ed., 2016, 55, 3058–3062 CrossRef CAS PubMed.
- X. Li, H. Zhang, Z. Mai, H. Zhang and I. Vankelecom, Energy Environ. Sci., 2011, 4, 1147–1160 CAS.
- S. Zhang, C. Yin, D. Xing, D. Yang and X. Jian, J. Membr. Sci., 2010, 363, 243–249 CrossRef CAS.
- Z. Yuan, Y. Duan, H. Zhang, X. Li, H. Zhang and I. Vankelecom, Energy Environ. Sci., 2016, 9, 441–447 CAS.
- W. Xu, Y. Zhao, Z. Yuan, X. Li, H. Zhang and I. F. Vankelecom, Adv. Funct. Mater., 2015, 25, 2583–2589 CrossRef CAS.
- B. Schwenzer, J. Zhang, S. Kim, L. Li, J. Liu and Z. Yang, ChemSusChem, 2011, 4, 1388–1406 CrossRef CAS PubMed.
- Z. Yuan, X. Li, Y. Zhao and H. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 19446–19454 CAS.
- Z. Yuan, X. Li, J. Hu, W. Xu, J. Cao and H. Zhang, Phys. Chem. Chem. Phys., 2014, 16, 19841–19847 RSC.
- D. Chen and M. A. Hickner, Phys. Chem. Chem. Phys., 2013, 15, 11299 RSC.
- H. Zhang, H. Zhang, X. Li, Z. Mai and J. Zhang, Energy Environ. Sci., 2011, 4, 1676 CAS.
- Y. Li, X. Li, J. Cao, W. Xu and H. Zhang, Chem. Commun., 2014, 50, 4596–4599 RSC.
- J. Cao, H. Zhang, W. Xu and X. Li, J. Power Sources, 2014, 249, 84–91 CrossRef CAS.
- J.-H. Kim and K.-H. Lee, J. Membr. Sci., 1998, 138, 153–163 CrossRef CAS.
- S. Liu, L. Zhou, P. Wang, L. Zhang, Z. Shao and B. Yi, J. Mater. Chem., 2012, 22, 20512–20519 RSC.
- A. K. Hołda, B. Aernouts, W. Saeys and I. F. Vankelecom, J. Membr. Sci., 2013, 442, 196–205 CrossRef.
- Y. Li, H. Zhang, H. Zhang, J. Cao, W. Xu and X. Li, J. Membr. Sci., 2014, 454, 478–487 CrossRef CAS.
- W. Xu, X. Li, J. Cao, H. Zhang and H. Zhang, Sci. Rep., 2014, 4, 4016 Search PubMed.
- Y. Li, H. Zhang, X. Li, H. Zhang and W. Wei, J. Power Sources, 2013, 233, 202–208 CrossRef CAS.
- P. Xing, G. P. Robertson, M. D. Guiver, S. D. Mikhailenko, K. Wang and S. Kaliaguine, J. Membr. Sci., 2004, 229, 95–106 CrossRef CAS.
- Q. Luo, H. Zhang, J. Chen, P. Qian and Y. Zhai, J. Membr. Sci., 2008, 311, 98–103 CrossRef CAS.
- H. Tsai, C. Kuo, J. Lin, D. Wang, A. Deratani, C. Pochat-Bohatier, K. Lee and J. Lai, J. Membr. Sci., 2006, 278, 390–400 CrossRef CAS.
- H. Zhang, H. Zhang, F. Zhang, X. Li, Y. Li and I. Vankelecom, Energy Environ. Sci., 2013, 6, 776–781 CAS.
- H. Chae Park, Y. Po Kim, H. Yong Kim and Y. Soo Kang, J. Membr. Sci., 1999, 156, 169–178 CrossRef CAS.
- P. Machado, A. Habert and C. Borges, J. Membr. Sci., 1999, 155, 171–183 CrossRef CAS.
- H. Matsuyama, M. Teramoto, R. Nakatani and T. Maki, J. Appl. Polym. Sci., 1999, 74, 171–178 CrossRef CAS.
- P. Van de Witte, P. Dijkstra, J. Van den Berg and J. Feijen, J. Membr. Sci., 1996, 117, 1–31 CrossRef CAS.
- X. Wei, Z. Nie, Q. Luo, B. Li, B. Chen, K. Simmons, V. Sprenkle and W. Wang, Adv. Energy Mater., 2013, 3, 1215–1220 CrossRef CAS.
- E. Agar, K. Knehr, D. Chen, M. Hickner and E. Kumbur, Electrochim. Acta, 2013, 98, 66–74 CrossRef CAS.
- B. Li, Q. Luo, X. Wei, Z. Nie, E. Thomsen, B. Chen, V. Sprenkle and W. Wang, ChemSusChem, 2014, 7, 577–584 CrossRef CAS PubMed.
- Q. Luo, L. Li, W. Wang, Z. Nie, X. Wei, B. Li, B. Chen, Z. Yang and V. Sprenkle, ChemSusChem, 2013, 6, 268–274 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18587h |
| This journal is © The Royal Society of Chemistry 2016 |





