Radioprotective effects of ultra-small citrate-stabilized cerium oxide nanoparticles in vitro and in vivo†
A. L. Popov *a,
S. I. Zaichkinaa,
N. R. Popovaa,
O. M. Rozanovaa,
S. P. Romanchenkoa,
O. S. Ivanovac,
A. A. Smirnova,
E. V. Mironovaa,
I. I. Seleznevaab and
V. K. Ivanovcd
*a,
S. I. Zaichkinaa,
N. R. Popovaa,
O. M. Rozanovaa,
S. P. Romanchenkoa,
O. S. Ivanovac,
A. A. Smirnova,
E. V. Mironovaa,
I. I. Seleznevaab and
V. K. Ivanovcd
aInstitute of Theoretical and Experimental Biophysics, Russian Academy of Sciences, Institutskaya 3, Pushchino, Moscow region, 142290 Russia. E-mail: antonpopovleonid@gmail.com; Tel: +7 4967 739148
bPushchino State Institute of Natural Sciences, Pushchino, Moscow region, 142290 Russia
cKurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, Moscow, Russia
dNational Research Tomsk State University, Russia
First published on 31st October 2016
Abstract
A comprehensive study of the radioprotective action mechanisms of ultra-small, citrate-stabilized, nanocrystalline cerium oxide (CeO2 nanoparticles, nanoceria) in vitro and in vivo was carried out. CeO2 nanoparticles were shown to significantly reduce the levels of hydrogen peroxide and hydroxyl radicals in aqueous solutions under X-ray irradiation. Pretreatment of primary mouse fibroblasts by CeO2 nanoparticles in a wide range of concentrations (10−5 to 10−9 M) protects cells from radiation-induced oxidative stress, maintaining a high level of dehydrogenase activity. A protective effect of CeO2 nanoparticles is also manifested in a decrease in the number of apoptotic cells and the level of reactive oxygen species (ROS) in a culture of primary mouse fibroblasts in vitro, as well as in a significant reduction (up to 50% of the control) of cytogenetic damage of mouse bone marrow in vivo. The intraperitoneal administration of CeO2 nanoparticles (200 nM) increased the rate of survival of mice exposed to X-ray radiation at a lethal dose, both in the prophylactic and the therapeutic schemes of administration, testifying the protection at the molecular, cellular and organism levels. The physical, chemical and biological origins of the protective action of CeO2 nanoparticles upon exposure of living systems to ionizing radiation in vitro and in vivo are discussed.
1. Introduction
Nanocrystalline cerium oxide (nanoceria) is widely used in various industrial applications, e.g. oxygen sensors, catalysts, automotive fuel additives.1–3 Most of today's nanoceria applications rely on the unique ability of cerium to switch easily between two oxidation states, namely Ce3+ and Ce4+.4 Numerous publications have shown great prospects for the application of CeO2 nanoparticles in biomedicine.5–8 It has been demonstrated, recently, that CeO2 nanoparticles are able to effectively protect living beings from the effects of chemical and radiation exposure.9–14 Several hypotheses have been put forward concerning the mechanisms of the protective action of nanoceria. Firstly, due to oxygen nonstoichiometry, CeO2 nanoparticles are able to participate in an unlimited number of redox–recovery–redox cycles, so that a single dose of nanoceria protects cells against free radicals more efficiently in comparison to the single or multiple application of vitamin E, n-acetylcysteine or melatonin.15,16 Secondly, it has been demonstrated that nanoceria can participate in the process of phosphorylation and regulate the activity of kinases,17 thus contributing to DNA repair and cell survival. However, the inconsistency and lack of experiments in vitro and in vivo does not allow an assessment of the contribution of each mechanism to the protective effect of CeO2 nanoparticles.Previously, it has been shown that there is a direct correlation between the antioxidant activity of CeO2 nanoparticles and their size.18 The reduction in size increases the oxygen non-stoichiometry of CeO2 nanoparticles, and, therefore, their antioxidant activity.19 In this regard, it can be assumed that the use of ultra-small cerium oxide nanoparticles (2–3 nm) would allow a reduction in the administration dose while maintaining high efficiency of treatment. Previously, we have proposed a very simple method for the synthesis of citrate-stabilized, aqueous, colloidal solutions of CeO2 nanoparticles of ultra-small size (∼2–3 nm), and shown their excellent biocompatibility and aggregative stability in neutral and mild alkaline media.20 Our present study is aimed at a comprehensive analysis of the radioprotective properties of citrate-stabilized CeO2 nanoparticles in vitro and in vivo.
2. Materials and methods
2.1. Preparation of cerium oxide nanoparticles
To prepare nanoceria sol, 0.24 g of citric acid was dissolved in 25 ml of a 0.05 M aqueous solution of cerium nitrate(III), rapidly poured into 100 ml of 3 M ammonia solution, with stirring, and allowed to stand for 2 h. The characteristics of the synthesized CeO2 nanoparticles were as follows: (1) core size: approximately 3–4 nm, and (2) zeta potential: −57 mV (ESI; Fig. S1 and S2†). For in vitro and in vivo experiments, nanoceria was resuspended in culture medium or in saline solution.2.2. X-ray irradiation
X-ray irradiation was conducted using an X-ray therapeutic machine RTM-15 (Mosrentgen, Russia) in a dose of 1 Gy for water solutions, 15 Gy for cell culture, 7 Gy for animals (survival test) and 1.5 Gy (micronucleus test), at a dose rate of 1 Gy min−1, 200 kV voltage, 37.5 cm focal length and 20 mA current.2.3. Animals
Two-month-old male SHK white outbred mice were used (24–26 g). Animals were maintained in a vivarium of the Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences, under standard conditions, and received a dry, standard ration and water ad libitum. Control animals were kept in identical cages and under the same standard conditions (20 ± 2 °C, 50 ± 10% relative humidity, a 12 h light/dark cycle) as the experimental animals. The experiments reported in this manuscript were approved by ethics committee of the Institute of Theoretical and Experimental Biophysics for the care and use of laboratory animals. The experiments conformed to the regulations and legal acts concerning the procedures of animal experiments and the humane treatment of animals (in accordance with Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes). The radioprotective effect of CeO2 nanoparticles in vivo was determined after intraperitoneal or intravenous injection of 10−6 M nanoceria sol at a rate of 8.3 nM g−1 of body weight. Each experimental group consisted of six mice for the micronucleus test, or 30 mice for the survival test.2.4. Cell culture
The radioprotective effect of CeO2 nanoparticles in vitro was studied using a primary culture of mouse fibroblasts. Fibroblasts were isolated from embryos of white outbred SHK mice on the 14th day of pregnancy, according to the standard protocol.21 Cells were seeded on 96-well plates at a density of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per cm2 in DMEM/F12 (1
000 per cm2 in DMEM/F12 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) supplemented with 5% FBS. After 6 h of cultivation, the medium in the wells was substituted by DMEM/F12 5% FBS with 10−5 to 10−9 M CeO2 nanoparticles. After 24 h, the cells were exposed to X-ray irradiation. Prior to irradiation, the medium in the wells was replaced by F12 (HEPES) without serum. Control samples were treated similarly to the experimental ones, without exposure to X-ray irradiation.
1) supplemented with 5% FBS. After 6 h of cultivation, the medium in the wells was substituted by DMEM/F12 5% FBS with 10−5 to 10−9 M CeO2 nanoparticles. After 24 h, the cells were exposed to X-ray irradiation. Prior to irradiation, the medium in the wells was replaced by F12 (HEPES) without serum. Control samples were treated similarly to the experimental ones, without exposure to X-ray irradiation.
2.5. Antioxidant activity of CeO2 nanoparticles
The antioxidant activity of CeO2 nanoparticles was determined by measuring the concentration of hydrogen peroxide and hydroxyl radical in bidistilled water, after X-ray irradiation. CeO2 nanoparticles (10−3 to 10−9 M) were added to the water just before irradiation. The concentration of hydrogen peroxide was determined by measuring the chemiluminescence intensity in the luminol–4-iodophenol–horseradish peroxidase, using a “Beta-1” liquid scintillation counter (Medapparatura, Ukraine) in a single photon counting mode.22 Determination of hydroxyl radical concentration was carried out using the reaction of hydroxyl radicals with coumarin-3-carboxylic acid.23 The fluorescence intensity was measured on a SFM 25A spectrofluorimeter (“Kontron Instruments”, Italy) at λex = 400 nm, λem = 450 nm, at room temperature.2.6. MTT assay
The activity of mitochondrial and cytoplasmic dehydrogenases of living cells was determined by the MTT assay, which is based on the reduction of colourless tetrazolium salt (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide).2.7. Live/Dead test
The numbers of live and dead fibroblasts were quantified using a Live/Dead BacLight Bacterial Viability Kit (Invitrogen), which utilizes mixtures of SYTO 9 green fluorescent nucleic acid stain (λ = 485/498 nm) and the red fluorescent nucleic acid stain (λ = 535/617 nm) propidium iodide (PI). These stains differ both in their spectral characteristics and in their ability to penetrate healthy cells. The SYTO 9 stain labels cells with both intact and damaged membranes. In contrast, PI penetrates only cells with damaged membranes, competing with the SYTO 9 stain for nucleic acid binding sites when both dyes are present. After 48 h of incubation, cells were washed with phosphate buffered saline three times, at room temperature. To perform the assay, 100 μL of combined Live/Dead assay reagents (containing approximately 1 μM SYTO 9 and 1 μM PI) were added to each well of the 96-well plate. The cells were incubated at room temperature for 20 min, then washed with phosphate buffered saline three times and observed under a fluorescence microscope Axiovert 200M (Zeiss). Images of fluorescently primary mouse fibroblasts were acquired using a digital camera (Canon A620). For each concentration of CeO2 nanoparticles, 15 fluorescent images of live cells and 15 fluorescent images of dead cells were taken. Cells were counted on each image using ImageJ software http://rsb.info.nih.gov/ij/. The data are presented as the ratio of live to dead cells, in percentage terms.2.8. Determination of ROS
The level of intracellular reactive oxygen species (ROS) was determined in vitro using 2′,7′-dichlorofluorescein diacetate (2,7-DCFH-DA). Cells were preincubated with CeO2 nanoparticles at various concentrations, for 24 h, in a 96-well black plate. The culture medium was replaced with Hanks solution containing 2,7-DCFH-DA (100 μM), and the cells were irradiated with X-rays. Untreated cells were used as a negative control. The fluorescence was detected using a Tecan 200 PRO plate reader (Tecan Corp., Switzerland).2.9. Apoptosis and micronucleus test
Changes in apoptotic cells in vitro were detected using fluorescence microscopy. Cells were stained for 10 min with Hoechst-33342 fluorescent dye (2.5 mg ml−1 in PBS). Observations were carried out using an Axiovert 200 microscope (Carl Zeiss, Germany) and the images were recorded using a Canon A620 digital camera (Canon, USA). The cytoprotective action of CeO2 nanoparticles in vivo was studied using the micronucleus test, which is one of the accepted standards in studies of cytogenetic damage to bone marrow in mice after exposure to ionizing radiation.24 Mice were irradiated with X-rays at a dose of 1.5 Gy 24 h after injection, and, after 28 h, they were euthanized. Cytological specimens were prepared according to standard procedures, with some in-house modifications.25 The criterion for cytogenetic damage was the percentage of polychromatic erythrocytes (PCE) with micronuclei (MN). For each experimental point, at least six animals were used, with 1000 PCEs in each experimental preparation and 3000 cells in the control.2.10. RT-PCR
Total RNA from the sample cells was isolated using a RNA extraction kit (SINTOL, Russia). Reverse transcription reaction was carried out using a kit for RT (SINTOL, Russia). Real-time PCR amplification was realized using a thermocycler ANK-32 (SINTOL, Russia). Primers used for estimation of transcription level for the analyzed gene and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (reference gene) were supplied by SINTOL. A kit (SINTOL, Russia) containing SYBR Green I intercalating dye was used for real-time PCR. The parameters of the PCR reaction were as follows: 95 °C – 5 min, followed by 40 cycles of: 95 °C – 30 s, 60 °C – 50 s. Then, the threshold cycle values obtained in PCR were determined. The sequences of primers used in real-time PCR were as follows:GPX-1 F: CCACCGTGTATGCCTTCT, R: GAGACGCGACATTCTCAATGA;
GSR F: AAAGAAGACCCCATCGGGCTCGGA, R: AGAGNAGGCAATCGACATCCGGAA;
CUZNSOD F: GTACCAGTGCAGGACCTCATTTT, R: GTCTCCAACATGCCTCTCTTCAT;
MNSOD F: CCACACATTAACGCGCAGAT, R: GGTGGCGTTGAGATTGTTCA;
GAPDH F: ATGTGTCCGTCGTGGATCTG, R: CCTGCTTCACCACCTTCTTG.
2.11. Survival test in vivo
CeO2 nanoparticles (8.3 nM g−1) were injected intraperitoneally (IP) or intravenously (IV), 15 min before or 15 min after X-ray irradiation. Then, surviving animals were counted once per day. The control involved two groups of mice: those injected with NaCl solution and irradiated at a dose of 7 Gy, and those not subjected to irradiation. Each experimental group consisted of 30 mice. The weighing of each animal and the estimation of water and food consumption was carried out every day.2.12. Statistical data analysis
Statistical analysis was performed using the methods of variation statistics. Mean values and the standard deviation of the mean were determined. The significance of differences between the groups in the micronucleus test was determined using the Student's t-test. The significance of differences in cell assays and experiments for animal viability was assessed using the U-Mann–Whitney criterion.3. Results
3.1. CeO2 nanoparticles inactivate hydrogen peroxide and hydroxyl radical formed by X-ray irradiation
We studied the effect of CeO2 nanoparticles on the formation of hydrogen peroxide (Fig. 1A) and hydroxyl radical (Fig. 1B) in water after exposure to ionizing radiation at a dose of 1 Gy.It was found that CeO2 nanoparticles, even at the lowest concentration (10−9 M), significantly reduced the level of hydrogen peroxide in irradiated samples, (by 20% of the control). An increase in CeO2 concentration (10−5 to 10−3 M) leads to a further reduction of hydrogen peroxide concentration, up to zero level. Thus, our findings confirm directly the ability of CeO2 nanoparticles to inactivate hydrogen peroxide generated upon X-ray ionization in vitro in water. It should be noted that nanoceria concentrations used in our experiments (10−6 to 10−9 M) were previously shown to be non-toxic to cells.26 Results obtained indicate also that CeO2 nanoparticles (10−3 M to 10−5 M) reduced the concentration of OH˙ in the water when exposed to X-rays at a dose of 1 Gy. The inactivation of highly active OH˙ by CeO2 nanoparticles could reduce the number of damaged macromolecules and cell membranes, preventing the development of necrosis and apoptosis processes.
3.2. CeO2 nanoparticles prevent cell death in primary culture of mouse fibroblasts after X-ray exposure
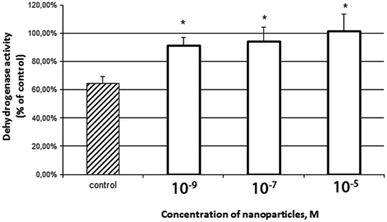
The goal of these experiments was to assess the viability of cells pretreated (24 h before irradiation) with different concentrations of CeO2 nanoparticles. A comparative analysis of the results using the MTT assay (Fig. 2) and the Live/Dead fluorescent test (Fig. 3) showed that the pretreatment of cells with CeO2 nanoparticles at concentrations of 10−5 to 10−9 M effectively protected cells from the damaging effects of ionizing radiation. The protective effect was maximal at a concentration of 10−5 M.3.3. CeO2 nanoparticles prevent the formation of micronuclei in primary culture of mouse fibroblasts after X-ray irradiation
A morphological analysis of the nuclear apparatus of the cells after exposure to ionizing radiation at a dose of 15 Gy revealed the following disorders in the nuclear apparatus and the appearance of apoptotic cell death features: reshaping of nucleus contours and the chromatin structure, dislocation of the nucleus to the periphery, including the formation of chromatin “clumps” (Fig. 4C and D). The number of disorder events in cells treated with CeO2 nanoparticles was significantly lower than in the irradiated control (Fig. 4D). The nuclear apparatus of non-irradiated cells was not damaged, irrespective of whether the CeO2 nanoparticles were added or not (Fig. 4A and B).The data obtained indicate that CeO2 nanoparticles do not cause any morphological changes in the nuclear apparatus, and prevent their formation upon X-ray irradiation at a dose of 15 Gy.
3.4. CeO2 nanoparticles reduce ROS generation after X-ray irradiation
An analysis was also carried out of the levels of ROS in cells using the fluorescent dye 2,7-DCFH-DA. Irradiation at a dose of 15 Gy increased the amount of ROS in primary mouse fibroblasts (Fig. 5), while preincubation of cells with CeO2 nanoparticles prevented the formation of ROS and greatly reduced their concentration in the whole range of CeO2 concentrations tested. This confirms that CeO2 nanoparticles act as an effective ROS scavenger in the cell. However, cerium oxide nanoparticles are not only an ROS scavenger, but also can modulate the expression of antioxidant enzymes and transcriptional factors involved in oxidative stress response.273.5. Protective action of CeO2 nanoparticles after X-ray irradiation in vivo
The results of cytogenetic damage studies in bone marrow cells are shown in Table 1. The study testified that a single injection of CeO2 nanoparticles prior to X-ray irradiation of animals led to a significant reduction in the amount of PCE with MN, compared to the control: by 51.74% in the case of intraperitoneal injection, and by 39.55% when CeO2 nanoparticles were administered intravenously.| Modes of treatment | Number of mice | Total number of PCEs | Number of PCEs with MN | PCEs with MN, % |
|---|---|---|---|---|
| a p < 0.05, compared to the control group at saline solution IV +1.5 Gy. | ||||
| Saline solution IV | 6 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
52 | 0.29 ± 0.03 |
| Saline solution IV +1.5 Gy | 6 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
758 | 6.32 ± 0.26 |
| CeO2 IV | 6 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
48 | 0.39 ± 0.07 |
| CeO2 IV +1.5 Gy | 6 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
688 | 3.82 ± 0.13a |
| Saline solution IP | 6 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
65 | 0.36 ± 0.04 |
| Saline solution IP +1.5 Gy | 6 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1098 | 6.10 ± 0.18 |
| CeO2 IP | 6 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
60 | 0.30 ± 0.02 |
| CeO2 IP +1.5 Gy | 6 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
366 | 3.05 ± 0.14a |
The animal life span after radiation exposure was chosen as the key parameter for investigating the radioprotective effect of nanoceria in vivo, being the main criterion of their radiosensitivity.28 The results are presented as survival rates (Fig. 6).
 | ||
| Fig. 6 The influence of CeO2 nanoparticles (10−6 M), before or after exposure to X-ray radiation at a dose of 7 Gy, on the survival rate of mice. | ||
The results obtained show that 90% of irradiated animals died on day 12 after irradiation. The injection of CeO2 nanoparticles 15 min before irradiation increased the survival rate of the animals, which was about 60% by the end of the experiment, confirming the ability of CeO2 nanoparticles to act as an effective radioprotective substance in vivo. Apparently, the survival rate could be increased by changing CeO2 nanoparticles' administration scheme. In the group of animals injected with CeO2 nanoparticles 15 min after irradiation, the survival rate by the end of the experiment was 40%.
To understand the molecular radioprotective mechanisms of CeO2 nanoparticles in vivo, we analyzed mRNA levels of some antioxidant enzymes and pro-inflammatory cytokines in the liver of mice after X-ray irradiation (Fig. 7). This experiment was inspired by previous reports concerning SOD mimetic activity and anti-inflammatory activity of CeO2 nanoparticles.29,30 The liver was chosen as the target organ because it accumulated the maximum amount of ceria upon intraperitoneal injection (Fig. S6†). Here, it is shown that X-ray irradiation at a dose of 1.5 Gy increased mRNA levels of CuZnSOD (9 fold) and MnSOD (15 fold), wherein pretreatment by intravenous injection of CeO2 nanoparticles significantly decreased mRNA levels of CuZnSOD and MnSOD in the liver. This effect can be attributed to SOD mimetic activity of CeO2 nanoparticles capable of inactivating ROS. It should be noted that application of CeO2 nanoparticles without further X-ray irradiation caused a slight increase in mRNA levels of antioxidant enzymes. Apparently, CeO2 nanoparticles act as a certain adaptive agent, which activates the antioxidant defence system of the cells, notably reducing cell damage upon exposure to ionizing radiation. The levels of proinflammatory cytokines are also modulated, but to a lesser extent. This can be explained by the fact that CeO2 nanoparticles hinder the development of oxidative stress, thereby blocking inflammation.
 | ||
| Fig. 7 Analysis of mRNA level (SOD1, SOD2, TNF-a, IL-6) in the liver, by real-time PCR, 24 h after intraperitoneal administration of CeO2 nanoparticles. Data are shown as mean ± SD with n = 3. | ||
4. Discussion and conclusions
The radiolysis of water caused by ionizing radiation results in the formation of high concentrations of hydroxyl radical (OH˙), which is very unstable, due to the presence of an unpaired electron, and has a short life time (several nanoseconds).31 The main mechanisms of hydroxyl radical formation in the cell are Fenton and Hardy–Weiss reactions. When affecting SH-groups, as well as histidine and other amino acid residues, OH˙ causes the denaturation of proteins and inactivates enzymes. OH˙ destroys carbohydrate linkages between nucleotides in nucleic acids and thus breaks DNA and RNA chains. Being a small and uncharged particle, it can penetrate into the hydrophobic lipid layer and react with polyunsaturated fatty acids that are part of biological membranes and lipoproteins of the plasma, which leads to their damage.32 In turn, hydrogen peroxide, which is one of the products of water ionization, also causes numerous structural and functional changes in the cell.33There are also long-term effects of exposure to ionizing radiation. It has previously been shown, by EPR spectroscopy, that, upon exposure of protein solutions and cells to ionizing radiation, long-lived protein radicals are formed34–36 with a half-life of 20 h or more.37,38 For instance, the formation of long-lived protein radicals was detected for egg albumin, bovine serum albumin, human serum albumin, lysozyme, immunoglobulin G and histone H1.39,40 It was found that long-lived protein radicals may be the source of continuous oxidative stress in biological systems.41 For example, gamma-radiation induces the generation of histone H1 radicals which are involved in the formation of DNA–protein crosslinks and oxidative damage of DNA bases, to form mutagenic products such as 8-oxoguanine.42 It was established that long-lived protein radicals cause mutations, leading to cell transformation.37 Therefore, free radicals and other ROS are formed not only during direct exposure to ionizing radiation, but also afterwards. In turn, our experiments have indicated that inactivation of ROS by CeO2 nanoparticles is not the only radioprotective mechanism of their action.
Our data have demonstrated the ability of ultra-small citrate-stabilized cerium oxide nanoparticles to inactivate hydroxyl radical and hydrogen peroxide, with high efficiency. These data have confirmed the chemical mechanism of protection provided by CeO2 nanoparticles against adverse effects of ionizing radiation (Fig. 8a). Our data have shown that the pretreatment of primary mouse fibroblasts by CeO2 nanoparticles protects the cells from a sublethal dose of X-rays. The higher concentration of CeO2 nanoparticles (10−5 M) provided almost 100% protection. Analysis of cytogenetic damage in vitro and in vivo has shown that CeO2 nanoparticles are able to prevent the formation of micronuclei in sublethal (15 Gy) and low doses (1.5 Gy) of ionizing radiation. Thus, the chemical mechanism of inactivation of radicals may not be the only possible explanation for the radioprotection of CeO2 nanoparticles. We hypothesize that, despite the ability to regulate the redox status within cells, CeO2 nanoparticles have a physical protective effect upon exposure to ionizing radiation, performing a shielding function. Early experimental data on the UV-shielding properties of CeO2 nanoparticles implicitly confirm our hypothesis.43–45 When analyzing our results and the data available from the literature, we can conclude that the direct physical protective effect of CeO2 nanoparticles on cell systems plays a significant role, along with inactivation of ROS and modulation of gene expression, upon exposure to ionizing radiation. Furthermore, there are interesting results concerning the ability of CeO2 nanoparticles to effectively absorb the radiation, including X-rays.46,47 Our results in vitro and in vivo allow an assumption to be made about the physical mechanism of a protective effect after X-ray irradiation (Fig. 8b).
Previously, it has been shown that the injection of CeO2 nanoparticles (7.5 μM) into mice before exposure to radiation (12.5 Gy) does not result in the death of animals within 60 days of the experiment.48 It has been shown that CeO2 nanoparticles (60 nM) as a radioprotective agent are more effective than amifostin, the most widely applied drug in modern clinical practice.49 Our study has shown that 10−5 to 10−3 M concentrations of CeO2 nanoparticles cause a significant decrease in the concentration of hydrogen peroxide and OH˙ radicals formed as a result of water radiolysis under the action of X-rays. However, it should be taken into account that the majority of free radicals and other ROS form directly during irradiation, and are rapidly destroyed.50 Given the concentration ratio of radicals and CeO2 nanoparticles (200 nM) in mice at the time of X-ray exposure, we can presume that the quantity of cerium oxide nanoparticles CeO2 nanoparticles is not quite sufficient to neutralize the pool of free radicals. Our results confirm the influence of CeO2 nanoparticles on repair processes after X-ray irradiation. DNA repair in irradiated cells takes several hours, or even days.51 Our finding that the injection of CeO2 nanoparticles after irradiation had a radioprotective effect in vivo supports the hypothesis of the involvement of CeO2 nanoparticles in the activation of the DNA repair system. These data are the first in the literature to show the effectiveness of CeO2 nanoparticles as a radioprotective agent administered therapeutically. The radiomodulating effect of CeO2 nanoparticles may be associated with their ability to influence post-radiation recovery in most radiosensitive organs and tissues, as well as to neutralize long-living active forms of proteins which are capable of generating secondary ROS for a long time after irradiation in vivo. In favour of this assumption is the fact that the activation of the MAP kinase signaling pathway and DNA polymerase-1 is a main molecular mechanism of damage repair after exposure to UV-irradiation.52 These facts allow us to make an assumption about the biological mechanism as a protective effect of ultra-small CeO2 nanoparticles after X-ray irradiation (Fig. 8c).
It has been shown that the pretreatment of intestinal epithelial cell cultures with CeO2 nanoparticles increases the expression of the SOD2 gene, and subsequent exposure to ionizing radiation resulted in a significant increase in cell survival.53 In this case, the early cellular sensor systems are activated in response to a change in the redox status of cells. One possible mechanism of this process might be realized via the ability of CeO2 nanoparticles to generate ROS, which serves as a substrate for SOD-2. In addition, the product of superoxide radical dismutation is hydrogen peroxide, which serves as an intracellular signaling messenger in nanomolar concentrations and is able to activate a number of transcription factors involved in cell proliferation, differentiation, migration, inflammation and cell death.54–56 CeO2 nanoparticles have also been shown to participate in the regulation of intracellular signaling pathways: ASK1-P38/JNK-NF-κB, MAPK, AP-1.57–59 This confirms the ability of CeO2 nanoparticles not only to cause direct effects, functioning as an antioxidant, but also to regulate, indirectly, the intracellular signaling system.
Several assumptions have been made regarding the mechanisms of complex radioprotective effects of CeO2 nanoparticles, and they have been confirmed, to some extent, by our research and by the works of other authors. On the one hand, the radioprotective effect of CeO2 nanoparticles may be associated with their ability to neutralize free radicals.60 On the other hand, CeO2 nanoparticles' action may be due to their participation in the process of phosphorylation and regulation of activity of ATM/ATR protein kinases, leading to the activation of the signaling cascade for repair of DNA double-strand breaks.61 The cytoprotective effects of CeO2 nanoparticles may also be due to their redox properties, causing the activation of genes for proteins involved in the antioxidant protection of cells (NFR-2, NF-KB) and ROS metabolism (catalase, SOD-1,-2, glutathione peroxidase 1,2,3).62,63
An increase in CeO2 radioprotection efficiency could be achieved by delivering it to the most radiosensitive organs, (thymus, spleen, bone marrow, intestines).
It is well known that pharmaceutics kinetics, biodistribution and clearance of CeO2 nanoparticles are influenced by many factors. The scheme (intravenously, intraperitoneally or orally) and the mode (once or periodically) of administration governs CeO2 nanoparticles' localization in organs and their clearance time. Yokel et al. (2013) studied the biodistribution of intravenously injected 5, 15, 30 or 55 nm cerium oxide nanoparticles in rats. Cerium oxide was found in blood, the brain, the liver and the spleen. The liver and the spleen contained most of the particles. No significant clearance was registered for more than 720 h.64 Portioli et al. (2013) studied the distribution of intravenously injected 10 nm cerium oxide nanoparticles 24 h after administration (1 or 20 mg kg−1). Consistent with previous investigations, the largest amount of cerium oxide nanoparticles was found to be accumulated in the liver and spleen, with some deposition also in the kidney and lungs; no signs of pathology of these organs were detected.65 Hirst et al. (2013) performed a comprehensive in vivo analysis of the biodistribution and antioxidant capability of cerium oxide nanoparticles administered to mice perorally (PO), intravenously (IV) or intraperitoneally (IP), by dosing animals weekly, for two or five weeks, with 0.5 mg kg−1 cerium oxide nanoparticles. They showed that the accumulation was maximal with IV and IP administration, while with PO administration mice excreted more than 95% of their cerium oxide nanoparticles within 24 h. Organ deposition for IV and IP administration was greatest in the spleen, followed by the liver, lungs and kidneys. In all the cases, ceria nanoparticles were excreted with feces. Cerium oxide nanoparticles administration showed no overt toxicity.66 Molina et al. (2014) studied tissue distribution, clearance and excretion of radioactive 141Ce after intratracheal instillation (IT), gavage or intravenous (IV) injection of neutron-activated 141CeO2 NPs and 141CeCl3 in Wistar Han rats. Risk from ingested nanoceria is likely to be far lower, due to very low absorption and rapid elimination of ceria which was not absorbed from the gastrointestinal tract. IV-Injected 141CeO2 NPs were predominantly retained in the liver, bone and spleen, which are all the organs that typically accumulate circulating particles. It has been suggested that cerium oxide nanoparticles are slowly cleared from the lungs, but have minimal extra pulmonary accumulation.67
Thus, the radioprotective properties of ultra-small, citrate-stabilized CeO2 nanoparticles are of significant interest, especially when considering multiple protective effects and the potential for successful application not only before, but even after, exposure to X-rays. However, the doses and modes of application require further investigation and clarification.
Acknowledgements
The study was supported by RFBR and Moscow City Government (joint grant 15-34-70019) and RFBR projects No. 14-44-03615, 16-34-60248. V. K. Ivanov is grateful for support from the Russian Science Foundation (project 14-13-01373).References
- N. Izu, W. Shin, I. Matsubara and N. Murayama, J. Electroceram., 2004, 13, 703–706 CrossRef CAS
.
- T. Masui, T. Ozaki, K. Machida and G. Adachi, J. Alloys Compd., 2000, 303, 49–55 CrossRef
.
- B. Park, K. Donaldson, R. Duffin, L. Tran, F. Kelly, I. Mudway, J. Morin, R. Guest, P. Jenkinson, Z. Samaras, M. Giannouli, H. Kouridis and P. Martin, Inhalation Toxicol., 2008, 20(6), 547–566 CrossRef CAS PubMed
.
- R. Robinson, J. Spanier, F. Zhang, S. Chan and I. Herman, J. Appl. Phys., 2002, 92, 1936–1941 CrossRef CAS
.
- V. Ivanov, O. Polezhaeva and Y. Tretyakov, Russ. J. Gen. Chem., 2010, 80, 604–617 CrossRef CAS
.
- N. Spivak, E. Shepel, N. Zholobak, A. Shcherbakov, G. Antonovitch, R. Yanchiy, V. Ivanov and Y. Tretyakov, Nano Biomed. Eng., 2012, 4(4), 188–194 CAS
.
- A. Popov, N. Popova, I. Selezneva, A. Akkizov and V. Ivanov, Mater. Sci. Eng., Proc. Conf., 2016, 68, 406–413 CrossRef CAS PubMed
.
- A. Shcherbakov, N. Zholobak, N. Spivak and V. Ivanov, Russ. J. Inorg. Chem., 2014, 59, 1556–1575 CrossRef CAS
.
- J. Chen, S. Patil, S. Seal and J. McGinnis, Nat. Nanotechnol., 2006, 1(2), 142–150 CrossRef CAS PubMed
.
- R. Tarnuzzer, J. Colon, S. Patil and S. Seal, Nano Lett., 2005, 5(12), 2573–2577 CrossRef CAS PubMed
.
- J. Colon, L. Herrera, J. Smith, S. Patil, C. Komanski, P. Kupelian, S. Seal, D. W. Jenkins and C. Baker, Nanomedicine, 2009, 5(2), 225–231 CAS
.
- P. Xu, B. Maidment, V. Antonic, I. Jackson, S. Das, A. Zodda, X. Zhang, S. Seal and Z. Vujaskovic, Radiat. Res., 2016, 185(5), 516–526 CrossRef CAS PubMed
.
- R. Madero-Visbal, B. Alvarado, J. Colon, C. Baker, M. Wason, B. Isley, S. Seal, C. Lee, S. Das and R. Mañon, Nanomedicine, 2012, 8(7), 1223–1231 CAS
.
- B. Rzigalinski, K. Meehan, R. Davis, Y. Xu, W. Miles and C. Cohen, Nanomedicine, 2006, 1(4), 399–412 CrossRef CAS PubMed
.
- M. Das, S. Patil, N. Bhargava, J. Kang, L. Riedel, S. Seal and J. Hickman, Biomaterials, 2007, 28(10), 1918–1925 CrossRef CAS PubMed
.
- D. Schubert, R. Dargusch, J. Raitano and S. Chan, Biochem. Biophys. Res. Commun., 2006, 342(1), 86–91 CrossRef CAS PubMed
.
- J. Niu, K. Wang and P. Kolattukudy, J. Pharmacol. Exp. Ther., 2011, 338, 53–61 CrossRef CAS PubMed
.
- W. Lijun, H. Wiesmann, A. Moodenbaugh, R. Kline, Y. Zhu, D. Welch and M. Suenaga, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 125415 CrossRef
.
- A. Shcherbakov, N. Zholobak, A. Baranchikov, A. Ryabova and V. Ivanov, Mater. Sci. Eng., C, 2015, 50, 151–159 CrossRef CAS PubMed
.
- O. Ivanova, T. Shekunova, V. Ivanov, A. Shcherbakov, A. Popov, G. Davydova, I. Selezneva, G. Kopitsa and Y. Tret'yakov, Dokl. Chem., 2011, 437(2), 103 CrossRef CAS
.
- J. Jozefczuk, K. Drews and J. Adjaye, J. Visualized Exp., 2012, 64, e3854 Search PubMed
.
- V. Bruskov, N. Popova, V. Ivanov, O. Karp, A. Chernikov and S. Gudkov, Biochem. Biophys. Res. Commun., 2014, 443(3), 957–961 CrossRef CAS PubMed
.
- N. Asadullina, A. Usacheva and S. Gudkov, J. Radiat. Res., 2012, 53(2), 211–216 CrossRef CAS PubMed
.
- A. Osipov, D. Klokov, A. Elakov, O. Rozanova, S. Zaichkina, G. Aptikaeva and A. Akhmadadieva, Nonlinearity Biol., Toxicol., Med., 2000, 2, 223–232 CrossRef PubMed
.
- S. Zaichkina, O. Rozanova, A. Dyukina, N. Simonova, S. Romanchenko, S. Sorokina, G. Aptikaeva and V. Yusupov, Biophysics, 2013, 58, 712–717 CrossRef CAS
.
- A. Popov, I. Selezneva, N. Kurnakov, V. Ivanov and P. Grigoriev, J. Biol. Phys. Chem., 2014, 14, 6–10 CrossRef
.
- G. Ciofani, G. Genchi, B. Mazzolai and V. Mattoli, Biochim. Biophys. Acta, 2014, 1840(1), 495–506 CrossRef CAS PubMed
.
- N. Asadullina, A. Usacheva, V. Smirnova and S. Gudkov, Nucleosides, Nucleotides Nucleic Acids, 2010, 29(10), 786–799 CAS
.
- E. Heckert, A. Karakoti, S. Seal and W. Self, Biomaterials, 2008, 29(18), 2705–2709 CrossRef CAS PubMed
.
- S. Hirst, A. Karakoti, R. Tyler, N. Sriranganathan, S. Seal and C. Reilly, Small, 2009, 5(24), 2848–2856 CrossRef CAS PubMed
.
- A. Ly, J. A. Aguilera and J. R. Milligan, Radiat. Phys. Chem., 2007, 76(6), 982–987 CrossRef CAS PubMed
.
- K. Takeshita, K. Fujii, K. Anzai and T. Ozawa, Free Radical Biol. Med., 2004, 36(9), 1134–1143 CrossRef CAS PubMed
.
- K. Vandepoele, D. Inzé, Y. Van de Peer and F. Van Breusegem, Mol. Biol. Evol., 2008, 25(3), 507–516 CrossRef PubMed
.
- R. Dean, S. Gieseg and M. Davies, Trends Biochem. Sci., 1993, 18(11), 437–441 CrossRef CAS PubMed
.
- V. Bruskov, O. Karp, S. Garmash, I. Shtarkman, A. Chernikov and S. Gudkov, Free Radical Res., 2012, 46(10), 1280–1290 CrossRef CAS PubMed
.
- S. Gudkov, I. Shtarkman, A. Chernikov, A. Usacheva and V. Bruskov, Dokl. Biochem. Biophys., 2007, 413, 50–53 CrossRef CAS PubMed
.
- S. Koyama, S. Kodama, K. Suzuki, T. Matsumoto, T. Miyazaki and M. Watanabe, Mutat. Res., 1998, 421(1), 45–54 CrossRef CAS PubMed
.
- T. Miyazaki, A. Morikawa, J. Kumagai, M. Ikehata, T. Koana and S. Kikuchi, Radiat. Phys. Chem., 2002, 65, 151–157 CrossRef CAS
.
- O. Karp, S. Gudkov, S. Garmash, I. Shtarkman, A. Chernikov and V. Bruskov Dokl, Dokl. Biochem. Biophys., 2010, 434, 250–253 CrossRef CAS PubMed
.
- N. Shtarkman, S. Gudkov, A. Chernikov and V. Bruskov, Biofizika, 2008, 53(1), 5–13 Search PubMed
.
- H. Ostdal, M. Davies and H. Andersen, Free Radical Biol. Med., 2002, 33, 201209 CrossRef
.
- E. Roberts and V. Deretic, Methods Mol. Biol., 2008, 445, 111–117 CAS
.
- K. Jung, SOFW J., 2013, 139(5), 1–14 Search PubMed
.
- F. Caputo, M. DeNicola, A. Sienkiewicz, A. Giovanetti, I. Bejarano, S. Licoccia, E. Traversa and L. Ghibelli, Nanoscale, 2015, 38, 15643–15656 RSC
.
- N. Zholobak, A. Shcherbakov, A. Bogorad-Kobelska, O. Ivanova, A. Baranchikov, N. Spivak and V. Ivanov, J. Photochem. Photobiol., B, 2014, 5(130), 102–108 CrossRef PubMed
.
- L. Levy, A. Pottier, A. Rouet, J. Marill, C. Devaux and M. Germain, Inorganic nanoparticles of high density to destroy cells in vivo, European Patent Application, EP 2 130 553 A1, 2009 Search PubMed
.
- Y. Zhang, P. Edmondson, T. Varga, S. Moll, F. Namavar, C. Lan and W. Weber, Phys. Chem. Chem. Phys., 2011, 13(25), 11946–11950 RSC
.
- M. Wason, J. Colon, S. Das, S. Seal, J. Turkson, J. Zhao and C. Baker, Nanomedicine, 2013, 9(4), 558–569 CAS
.
- C. Baker, Transl. Cancer Res., 2013, 2(4), 343–358 CAS
.
- Y. Hosokawa and T. Sano, Radiat. Prot. Dosim., 2015, 167(1–3), 326–330 CrossRef PubMed
.
- M. Anikina and N. Mikhailov, Radiobiology, 1991, 31(1), 71–76 Search PubMed
.
- N. Purohit, M. Robu, R. Shah, N. Geacintov and G. Shah, Sci. Rep., 2016, 12(6), 19020 CrossRef PubMed
.
- J. Colon, N. Hsieh, A. Ferguson, P. Kupelian, S. Seal, D. W. Jenkins and C. Baker, Nanomedicine, 2010, 6(5), 698–705 CAS
.
- G. Buettner, B. Wagner and V. Rodgers, Cell Biochem. Biophys., 2013, 67, 477–483 CrossRef CAS PubMed
.
- J. Boonstra and J. Post, Gene, 2004, 337, 1–13 CrossRef CAS PubMed
.
- E. Kairuz, Z. Upton, R. Dawson and J. Malda, Wound Repair Regen., 2007, 15, 266–274 CrossRef PubMed
.
- X. Cai, S. Seal and J. McGinnis, Biomaterials, 2014, 35(1), 249–258 CrossRef CAS PubMed
.
- G. Cheng, W. Guo, L. Han, E. Chen, L. Kong, L. Wang, W. Ai, N. Song, H. Li and H. Chen, Toxicol. In Vitro, 2013, 27(3), 1082–1088 CrossRef CAS PubMed
.
- S. Lung, F. Cassee, I. Gosens and A. Campbell, Inhalation Toxicol., 2014, 26(10), 636–641 CrossRef CAS PubMed
.
- A. Asati, S. Santra, C. Kaittanis and J. Perez, ACS Nano, 2010, 4(9), 5321–5331 CrossRef CAS PubMed
.
- N. Zholobak, V. Ivanov, A. Shcherbakov, A. Shaporev, O. Polezhaeva, A. Baranchikov, N. Spivak and Y. Tretyakov, J. Photochem. Photobiol., B, 2011, 102, 32–38 CrossRef CAS PubMed
.
- V. Selvaraj, N. Nepal, S. Rogers, N. Manne, R. Arvapalli, K. Rice, S. Asano, E. Fankhanel, J. Ma, T. Shokuhfar, M. Maheshwari and E. Blough, Biomaterials, 2015, 59, 160–171 CrossRef CAS PubMed
.
- L. Rubio, B. Annangi, L. Vila, A. Hernández and R. Marcos, Arch. Toxicol., 2016, 90(2), 269–278 CrossRef CAS PubMed
.
- R. Yokel, M. Tseng, M. Dan, J. Unrin, U. Graham, P. Wu and E. Grulke, Nanomedicine, 2013, 9(3), 398–407 CAS
.
- C. Portioli, D. Benati, Y. Pii, P. Bernardi, M. Crucianelli, S. Santucci, M. Bentivoglio and M. Passacantando, Nanosci. Nanotechnol. Lett., 2013, 5(11), 1174–1181 CrossRef CAS
.
- S. Hirst, A. Karakoti, S. Singh, W. Self, R. Tyler, S. Seal and C. Reilly, Environ. Toxicol., 2013, 28(2), 107–118 CrossRef CAS PubMed
.
- R. Molina, N. Konduru, R. Jimenez, G. Pyrgiotakis, P. Demokritou, W. Wohllebenb and J., Environ. Sci.: Nano, 2014, 1, 561 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18566e |
| This journal is © The Royal Society of Chemistry 2016 |