Large dopant dependence of the current limiting properties of intrinsic conducting polymer surge protection devices
Abstract
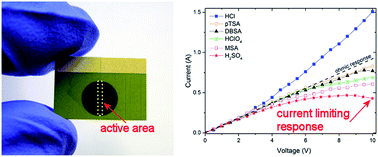
New two terminal surge protection devices based on intrinsic conducting polymers are demonstrated to be strongly affected by the dopant molecule type. Thermogravimetric analysis combined with current–voltage studies show a causal link between the dopant molecule, moisture content and the current limiting capability of the devices. Polyaniline thin-films with high moisture content produce devices with current saturation and foldback effects at high applied voltages while low moisture content films exhibit no current rectification and instead demonstrate decreasing resistivity with increasing voltage. Polyaniline doped with sulfuric acid (H2SO4) exhibited the largest moisture content and surge protection devices built with this material produced for the first time negative differential resistance under ambient conditions. A further improvement was made upon this through surface engineering of the interface between the polymer and electrodes using self-assembled monolayers.


 Please wait while we load your content...
Please wait while we load your content...