N-Heterocyclic carbene copper(i) complex-catalyzed synthesis of 2-aryl benzoxazoles and benzothiazoles†
Abstract
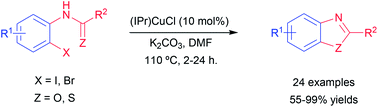
A new and efficient synthesis of 2-arylbenzoxazoles and benzothiazoles using a copper N-heterocyclic carbene complex is described. In a simple protocol a variety of 2-substituted benzoxazoles and benzothiazoles were obtained via intramolecular coupling cyclization of 2-haloanilides/2-halothioanilides in good to excellent yields.

 Please wait while we load your content...
Please wait while we load your content...