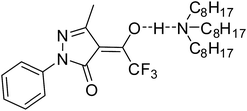
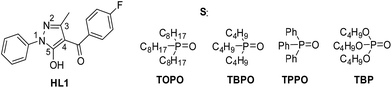
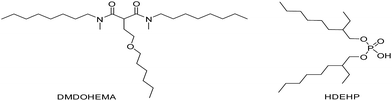
The interaction of extractants during synergistic solvent extraction of metals. Is it an important reaction?
Maria Atanassova*a,
Vanya Kurtevab and
Ivan Dukova
aUniversity of Chemical Technology and Metallurgy, Department of General and Inorganic Chemistry, 8 Kliment Okhridski blvd., 1756 Sofia, Bulgaria. E-mail: ma@uctm.edu
bInstitute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Acad. G. Bonchev street, Block 9, BG 1113, Sofia, Bulgaria
First published on 15th August 2016
Abstract
The enhancement of the synergistic solvent extraction of metallic species may depend on the nature and strength of the possible interaction between the two ligands. The prediction of the extraction systems properties remains a difficult task due to a lack of knowledge about the behaviour of the acidic/neutral or acidic/cationic couples. The use of multiple analytical techniques (NMR, FT-IR, UV-vis, ESI-MS) gives more insight into the structure of the formed adducts in the organic phase responsible for the weakened extraction process and destruction of synergism. The increased acidity of the acidic extractant and the basicity of the neutral ligand lead to a stronger tendency to react with each other forcing the consumption of extractants liable for antisynergism. Major conclusions on the role of such intermolecular interactions towards the extraction mechanism and yields are additionally provided. This information could be useful nowadays when developing new synergistic extraction systems includes ionic liquids as innovative organic media instead of VOCs, despite the complicated chemical environment provided by these modern alternative diluents. This article gives an overview on some investigations as well as on our own contributions on this topic.
1 Introduction
A liquid–liquid or solvent extraction system is composed of two immiscible liquid phases that are unlimitedly free to mix on agitation.1 The organic medium usually contains lipophilic complexing agents that promote distribution of the analyte into the organic phase by the formation of lipophilic complexes or ion pairs.2,3 Organic chemists have rendered yeoman's service by synthesizing an array of ligands with different functional groups containing various donor atoms (P, S, N etc.).4 The diluent should not be too volatile especially if equilibration takes a lot of time and the room is warm as well as it is best if the diluent is immiscible with water. The major allotment of success for the extraction procedure goes to extraction by chelation. Rare-earth extractions by acidic reagents are greatly influenced by a cation-exchange mechanism, where H+ is exchanged for the cation of interest. The main difference between β-diketones and organophosphoric acids is the fact that β-diketones are mostly monomeric in organic solutions. The properties of a variety of systems of extractants are described with special emphasis on the β-diketones, which are thoroughly investigated ligands. By judicious choice of solvating and acidic compounds, the resulting distribution ratio will be greater than the sum of either extractant functioning alone.5–7 Aforesaid solvating molecule improves the extraction by rendering the complex more lipophilic. It accomplishes this either by expending the coordination sphere, or by replacing water molecules in the first coordination sphere of the metal ion. This phenomenon of greatly enhanced extraction i.e. synergism due to a mixture of extractants has attracted considerable attention since its discovery in 1958.8Most of the examples illustrating synergism use a chelating agent, which neutralizes the charge on the metal plus an active donor synergistic agent. The synergistic effect of neutral organophosphorus derivatives in the extraction of metal ions with β-diketones has been the subject of a large number of papers and reviewers, and is generally interpreted by the formation of mixed complexes in the organic phase:
| Mn+ + nHA + xS ⇌ MAn·Sx + nH+, |
The enhancement of the distribution ratio can be as high as 106 and is attributed to the higher solubility of the synergistic adduct in the organic phase. The most interesting question, however, is how the neutral reagent (S) is coordinated in the mixed complex: directly to the metal with or without increase in the coordination number (CN), causing the bidentate chelate ligand to become monodentate, an directly to the chelate through the adduct donor electrons.9,10 The advent of new chelating compounds has been brought about further discussion of a rule relating the stability of a metal chelate and the stability of its adducts. Can someone generalizes and say that the stronger acid is a reagent, the more stable adducts it forms and the larger the synergistic effect in solvent extraction is? Synergistic enhancement is expected not only for metal complexes “coordinately unsaturated”. Metals with coordination number at least twice their charges were indeed reported to be involved in synergistic extractions, e.g. 4f and 5f ions. Furthermore, if the ionic radius of the central metal ion is too small, the attachment of a new ligand may become impossible. The most significant features of the f-electron cations in aqueous solution are the stability of the trivalent state and the strongly ionic character of their bonding. The strong ionic nature of the bonding allows to reach variable coordination numbers (CN = 6 to 12) in their complexes of 4f and 5f ions, but in solution CNs of 8 and 9 are rather common.11,12 Because of their high CNs, these metal cations usually have a water of hydration remaining attached to the inner coordination sphere even on complexation with polydentate ligands. Addition of a second ligand results in the replacement of the remaining water molecules by formation of a ternary complex.10,13 In fact the extraction of mixed synergistic complexes is not limited to the case of actinoids or rare earth elements.14 An attempt is also made in order to establish which mixtures of extracting agents exhibit the highest synergistic effect and which are the best experimental conditions for the occurrence of this effect. Owing to the diversity of extractant combinations causing synergism, the Healy's classification is widely adopted, namely: acidic (anionic) plus neutral ligands; two acidic extractants; cationic plus neutral molecules; cationic plus anionic compounds15,16 and two cationic reagents. The first system acidic/neutral duo is the simplest and best understood up to date. These systems usually behave ideally under a variety of conditions, and the experimental data can relatively easily be interpreted in terms of simple mass action equations. The reverse of synergism, i.e. antagonism or antisynergism was noted by Blake17 and Peppard18 in the alkyl-phosphoric acid–phosphorus ester system as well as by Healy et al.19 in the system HTTA–TBP (thenoyltrifluoroacetone/tributyl phosphate) when the second extractant is added in excess. Ferraro and Peppard20 investigated the nature of interaction between TBP and mono-(2-ethylhexyl)-phosphoric acid (H2MEHP) by the use of several techniques including infrared and cryoscopic studies and isopiestic measurements. The results have given evidence that an association product had resulted at a ratio of 2 moles of TBP per hexamer unit of H2MEHP. It appears that excess of the donor solvent (S) reduces the concentration of free chelating agent by increasing interaction between the acid and S through hydrogen-binding as a result of which the extraction ratios become smaller. In the second example, destruction of synergism is related to the water content of the organic phase and the destruction of the anhydrous synergistic species M(TTA)xSy. It is remarkable that throughout both the synergism and antagonistic stages, the M(TTA)x chelate remains an entity in the organic phase.21 It has been found that sometimes the increase of one extractant's concentration and keeping the other constant, causes initially an increase of the distribution ratios (synergistic region) and then – their decrease (destruction of synergism region). Marcus and Kertes21 have noted that a direct interaction between the extractants has an appreciable effect on the breakdown of the synergism, but later studies have shown that both phenomena (synergism and antagonism) are more complex.8,10,13,22–27 The synergistic solvent extraction of all lanthanoid(III) ions with a ternary mixture of 4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one (HP) or HTTA in combination with the quaternary ammonium salt, ionic liquid methylyrialkyl (C8–C10) ammonium chloride (Aliquat 336, QCl) in C6H6 has been investigated by Atanassova et al. and the anionic complex Q[LnP3(TTA)] was established.28 However, a synergistic effect was observed for light 4f-ions only (La, Ce, Pr, Nd and Sm) while for Eu(III) and Gd(III) and the heavier members of the 4f-series an antagonistic effect was found. The presence of the molecule–ion interaction between ethylenediaminetetraacetic acid disodium salt (Na2H2EDTA) and β-cyclodextrin (CD) was revealed by Song and co-researchers29 leading to a decreased coordination interaction of Na2H2EDTA and copper chloride. A possible explanation was proposed by authors that there was a competitive relationship between the molecule–ion and the coordination interaction. Further, nuclear magnetic resonance measurements provided an important information on the difference in interaction modes of β-CD with H2EDTA2− and [Cu(EDTA)]2−.
One of the major drawbacks of solvent extraction chemistry nowadays is the generation of great volumes of contaminated diluents.30,31 Considerable work has been done in recent years in order to replace VOCs, often toxic with novel more eco-friendly alternatives like ionic liquids (ILs) as extractive diluents. In the beginning of the 21st century, the emergence of newfangled air- and water stable ILs marked up significantly their plausible application for the extraction of valuable constituents and precious metals and further lead to improved extraction performances.32–35 The interactions between the anions of ionic liquids and metal ions are widely studied, mainly in respect for their solvation.36 On the other hand, it has been found in the extraction of natural compounds37–40 that the interactions between the extracted species and ILs are crucial for efficient processes. It has also been demonstrated41 that the interactions between ILs and radicals play an important role in the radical reactions in ionic liquids. An isolated research work is the potential interaction, studied by NMR analysis, among imidazolium based ionic liquids (1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl) amide, RmimTf2N, n = 4, 6, 8, 10), and some commonly applied acidic/neutral molecules in solvent 4f and 5f-ions extraction recently presented by some of us.36 The examination of the solvent–solute interactions (types and strengths) is unavoidable for a better understanding of the chemical mutual solubility (aqueous-biphasic systems), reactivity and selectivity because ILs have a strong imprint on the mechanism of pick-up metal ions.42 The experimental results show that no IL–ligand interactions occurred in chloroform solution independently on the length of the imidazolium alkyl chain or on the structure and acidity of the ligand. On the other hand, in [Tf2N]− based ionic liquids, the cyclic voltammograms of uranium(IV) hexachloro complex present two redox couples (UVCl6−/UIVCl62− and UIVCl62−/UIIICl63−)43 and IL cations interact with the anionic uranium species. The magnitude of the ion pairing was evaluated by voltammetry and ab initio calculations. It was established by Cannes and co-researchers that the interaction increases with the charge of the uranium complex, UVCl6− < UIVCl62− < UIIICl63−, and depends on the IL cation nature, [MeBu3N]+ < [BuMe2Im]+ ≈ [BuMePyr]+ < [BuMeIm]+. The dependence of metal extraction and Zn(II)/Cu(II) separation on intermolecular interactions between di(2-ethylhexyl)phosphoric acid (P204) and ILs (trihexyl(tetradecyl)phosphonium chloride or methyltrioctylammonium chloride) have been elucidated by Li et al.44 The IR and NMR spectroscopies clearly reveal the diverse interactions between P204 and ILs, which can be adjusted by changing the composition of mixed extractants, thus causing the different degree of antagonistic effect on extraction process. Actually, additional extraction studies are of crucial importance to clearly identify the intermolecular interaction impact through the extraction mechanism.
The major part of the present overview will be devoted to the mixtures containing acidic (anionic) plus cationic/neutral extractants. The following discussion is divided into two distinctive sections regarding the interaction between acidic compound in combination with amines (part one) or neutral organophosphorus compound families, (subdivision two). The acidic compound can be a β-diketone, another chelating agent or organophosphoric acid. Relatively few works were devoted in the past to the destruction of synergism and even less papers have been published on the subject in recent years. This review covers the literature until the end of May 2016, and to the best of our knowledge no review articles high-lighting this specific topic in the field of solvent extraction chemistry have been previously published.
2 Interaction between acidic (chelating) agents and various ammonium salts
In the last six decades a great deal of interest has been focused on the extraction of metal ions with combinations of chelating extractants and alkylammonium salts. It has been established that for many systems the synergistic enhancement is very high (up to 105 to 106). Often β-diketones like thenoyltrifluoroacetone (HTTA), acetylacetone (ACAC), trifluoroacetylacetone (HTFA), benzoyltrifluoroacetone (HBTFA) etc., derivatives of acylpyrazolone family (most often 1-phenyl-3-methyl-4-benzoyl-pyrazol-5-one, HPMBP) have been used as chelating extractants. On the other hand, various kinds of alkylammonium salts (primary, secondary, tertiary and quaternary) have been applied as synergistic agents. Since the chelating extractants and alkylammonium salts are weak acids and bases, they could interact with each other and thus to influence on the synergistic reaction. Newman and Klotz have studied the synergistic solvent extraction of Th(IV), Am(III) and Ce(III) with mixtures of HTTA and tri-n-octylamine (TOA).45–50 They have determined that in the presence of HCl the extractants interact with each other forming the compounds TOAHCl, HTTA·TOAHCl and HTTA·TOA.45,46 The authors have concluded that these three species contribute equally to the synergistic effect in the extraction of Am and Ce and as consequence they have been bound directly to Am in Am(TTA)3.47,48 However, when Th(IV) has been extracted with mixtures of HTTA and TOA the complex Th(TTA)4TOAHCl has been formed and the species HTTA·TOAHCl and HTTA·TOA haven't contribution to the synergistic enhancement. The authors have accepted that in this particular case TOAHCl is attached to one of HTTA molecule and not to Th(IV) itself.49,50 The conclusions of Newman and Klotz have not been confirmed by Ke and Li.51,52 The authors have studied spectrophotometrically the adduct formation of the chelates Cu(TTA)2 and Cu(TFA)2 with tri-n-butylamine (TBA), tri-n-hexylamine (THA), tri-n-octylamine (TOA), tri-n-laurilamine (TLA) and their corresponding hydrochloride salts. The obtained visible and IR spectra have shown that the alkylamines are bound to Cu(II) through their nitrogen atoms until the alkylamine hydrochlorides are bound to Cu(II) through their chlorine atoms. The calculated values of the equilibrium constant for adduct formation with R3NHCl are much greater than those for R3N. For example, these values for Cu(TTA)2–TOA and Cu(TTA)2–TOAHCl are 21.4 ± 1.3 and 781 ± 21 and those for Cu(TFA)2–TOA and Cu(TFA)2–TOAHCl are 12.5 ± 0.5 and 936 ± 43, respectively. The main reason for the author's explanation is that the bulky R groups in R3NHCl are far away from the bonding site. So, the steric effects are smaller. In addition, the experimental data have shown that the species HTTA·TOA and HTTA·TOAHCl do not act as synergistic agents which is in contrast with the results of Newman and Klotz. The solvent extraction experiments for the synergistic extraction of Co(II) and Zn(II) with HTTA and TOA or TOAHCl have confirmed these conclusions.52 It should be also remarked that Genov and Dukov have studied the synergistic extraction of Pr(III), Gd(III) and Yb(III) with mixtures of HTTA and TOAHCl in 1973.53 Formation of adducts Ln(TTA)3TOAHCl has been established in accordance with the results of Ke and Li. Investigation of the extraction of Pr with binary mixtures (HTTA–DOAHCl (di-n-octylamine hydrochloride), S1) and HTTA–TOAHCl, S2, as well as with ternary mixtures (HTTA–DOAHCl–TOAHCl) has been performed in order to establish possible interactions between HTTA and the two tertiary amines.54 The experimental data are shown in Table 1.| Extractant | Concentration | DT,S1 | DT,S2 | DT,S1 + DT,S2 | DT,S1,S2 |
|---|---|---|---|---|---|
| HTTA | 5.0 × 10−2 M | 0.43 | 0.14 | 0.57 | 0.53 |
| [S1] = [S2] = 2.5 × 10−3 M | 6.5 × 10−2 M | 0.90 | 0.28 | 1.18 | 1.10 |
| 8.0 × 10−2 M | 1.58 | 0.50 | 2.08 | 2.09 | |
| 9.6 × 10−2 M | 2.63 | 0.90 | 3.53 | 3.47 | |
| DOAHCl (S1) | 2.5 × 10−3 M | 1.55 | 0.50 | 2.05 | 1.95 |
| [HTTA] = 8.0 × 10−2 M | 3.75 × 10−3 M | 2.20 | 0.50 | 2.70 | 2.70 |
| [S2] = 2.5 × 10−3 M | 5.0 × 10−3 M | 2.88 | 0.50 | 3.38 | 3.38 |
| 7.5 × 10−3 M | 4.00 | 0.50 | 4.50 | 4.67 | |
| TOAHCl (S2) | 2.5 × 10−3 M | 1.55 | 0.50 | 2.05 | 2.00 |
| [HTTA] = 8.0 × 10−2 M | 3.75 × 10−3 M | 1.55 | 0.70 | 2.25 | 2.00 |
| [S1] = 2.5 × 10−3 M | 5.0 × 10−3 M | 1.55 | 0.89 | 2.44 | 2.00 |
| 7.5 × 10−3 M | 1.55 | 1.26 | 2.81 | 2.00 |
It is seen that for the first two sets of experiments the sum (DT,S1 + DT,S2) is practically equal to the values of DT,S1,S2. It is fulfilled even when the concentration of one of the extractants is increased up to 3 times keeping the concentrations of the other two constant. These results were interpreted as absence of interaction between the extractants at the applied experimental conditions, because according to the Le Chatelier's principle the increase of the concentration of one of the extractants would shifted the equilibria below
| HTTA + DOAHCl ⇌ HTTA·DOAHCl |
| HTTA + TOAHCl ⇌ HTTA·TOAHCl |
| HTTA + DOAHCl + TOAHCl ⇌ HTTA·DOAHCl + HTTA·TOAHCl + HTTA·DOAHCl·TOAHCl |
In order to correlate qualitatively the interaction in different binary mixtures the FT-IR spectra for organophosphorus acids (di-2-ethylhexyl phosphoric acid (D2EHPA), 2-ethylhexyl phosphoric acid mono-2-ethylhexyl ester (PC88A) and 2,2,4-trimethylpenthyl phosphinic acid (Cyanex 272)) and trioctyl/decyl amines (Alamine 336 and TEHA (triethylhexylamine)) and their mixtures have been analysed by Liu and Lee56 in n-hexane, xylene and toluene at fixed 0.5 M concentrations. The change in the intensity of some characteristics bands, like P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, P–O, and C–N, and their appearance/disappearance made it possible to identify the interaction degree occurring in the organic phase. In the binary mixtures, the organophosphorus acid acts as a proton donor, while the tertiary amine has unshared electron pair on nitrogen atom and the possible interaction can be represented as: HA + R3N ↔ R3NHA. Since the acidity of the organophosphorus acid extractants changes in the order: D2EHPA > PC88A > Cyanex 272, it can be concluded that the interaction between amine and acid is proportional to the acidity of the acid ligands as its proton donor tendency increases. Therefore, D2EHPA showed the strongest tendency to react with Alamine 336 among the three acidic molecules. The FT-IR spectra have shown that in diluents with low dielectric constant, the interaction between extractants prevails while in diluents with relatively high dielectric constant the interaction between the acidic molecule and diluent is more important.57
O, P–O, and C–N, and their appearance/disappearance made it possible to identify the interaction degree occurring in the organic phase. In the binary mixtures, the organophosphorus acid acts as a proton donor, while the tertiary amine has unshared electron pair on nitrogen atom and the possible interaction can be represented as: HA + R3N ↔ R3NHA. Since the acidity of the organophosphorus acid extractants changes in the order: D2EHPA > PC88A > Cyanex 272, it can be concluded that the interaction between amine and acid is proportional to the acidity of the acid ligands as its proton donor tendency increases. Therefore, D2EHPA showed the strongest tendency to react with Alamine 336 among the three acidic molecules. The FT-IR spectra have shown that in diluents with low dielectric constant, the interaction between extractants prevails while in diluents with relatively high dielectric constant the interaction between the acidic molecule and diluent is more important.57
The synergistic solvent extraction of divalent and trivalent transition metal ions (Fe, Co, Cu, Zn) with mixtures of HTTA and several amines in CHCl3 has been investigated by Aly et al.58–61 The extraction of different cations was found to increase by more than three order of magnitude in the presence of dibenzylamine in chloroform.58 Eight alkyl and arylamines have been used for Co(II) extraction and in addition, UV and IR spectra have shown that the amines are bound directly to the metal.60 Saeed and co-reserchers62–67 have studied the synergistic extraction of trivalent lanthanoids(III), Fe(III) and Mn(II) from perchlorate media with HTTA in combination with tribenzylamine (TBnA) in chloroform. The stoichiometric composition of the synergistic complexes was determined as Ln(TTA)3·3TBnA (Ln = Pr, Sm, Eu, Tb, Ho, Er, Yb and Lu), Fe(TTA)3·TBnA and Mn(TTA)2·2TBnA. The results have demonstrated antisynergistic effect from pH 3 to 6 in the extraction of Lu(III). To this aim, the change in the CN of the synergistic adduct of Lu(III) at different pHs was further investigated through thermodynamic functions like enthalpy, entropy and Gibbs free energy.60 On the basis of spectral analysis of HTTA and TBnA solutions as well as their mixed solutions it was concluded that there is no interaction between them.63
Still in 1971 Genov et al.68 have studied the solvent extraction of Eu(III) with HTTA and the IL Aliquat 336 (QCl). Extraction of anionic complex Q+[Eu(TTA)4]− was found and the important role of the quaternary ammonium salt anion was established. The change of Cl− with NO3− and ClO4− caused a significant decrease in the Eu extraction (up to 3–4 orders of magnitude). After this proof or principle the investigations of the factors influencing the lanthanoid extraction with HTTA and Aliquat 336 in chloride and perchlorate forms have been carried out by Dukov et al. and further several works appeared.69–72 The synergistic extraction of the entire series of lanthanoids (without Ce and Pm) has been studied lately by Atanassova et al. and represents a strong contribution too.70 The formation of anionic complexes Q+[Ln(TTA)4]− was explained by the breaking of the bond between the cation and anion of the quaternary ammonium salt. The large decrease of the synergistic enhancement for QClO4 (ref. 71) has been interpreted with the stronger bond between the cation and the anion in QClO4 than those in QCl. The four anions of the chelating extractant (TTA−) form the inner coordination sphere of the complex satisfying the coordination abilities of the lanthanoid ion (CN = 8). The cation of the salt Q+ occupies the outer sphere of the complex. An isolated research work of Khopkar and Mathur, where formation of complexes [M(TTA)3Cl]−Q+ was reported for the extraction of Am, Cm, Eu, Tb and Lu with mixtures of HTTA and Aliquat 336 in CHCl3 have not been confirmed later.73
Anionic complexes of the same type Q+[Mn+Ln+1] (L− is a chelating extractant) have been affirmed by Sekine et al.74–81 for the extraction of mono-, di- and trivalent metal ions with combinations of (HBnTFA) or HTTA and tetrabutylammonium ions (TBA+). The authors have accepted that the ion-pairs TBA+BnTFA− or TBA+TTA− result of interaction between the ligands take part in the formation of these anionic complexes. However, no proves for the ion-pairs formation have been evidenced by additional experiments and conventional methods. The coordination of the ion-pair to the metal ion has not been commented, too. In addition, interaction between HTTA and tri-n-octylmethylammonium chloride (capriquat) has been reported by Inoue et al.82 for the extraction of Np(V) and this group of researchers have ascertained that these intermolecular reaction seriously affected the extraction process.
Various acylpyrazolone compounds have been used for the solvent extraction of almost all metal ions alone and in combinations of alkylamines or quaternary ammonium salts. The pKa values of 4-acyl-5-pyrazolones are between 2.5 and 4.0,25 so they are more acidic than the popular β-diketone HTTA largely employed. Hence, it could be admitted that the possible interaction implementing cationic extractants could be stronger than that between β-diketones and alkylammonium salts. However, there are too much opposite opinions for the impact of extractant's interaction. Freiser et al.83–85 have reported data for the extraction of La, Pr, Eu and Yb with several acylpyrazolones and methyltriheptylammonium or methyltrioctylammonium chlorides. In all cases formation of anionic complexes has been established, but unfortunately the interaction between extractants has not been commented. In several papers Brunette and co-workers86–93 have observed that at definite conditions the chelating extractants can react with the amine salts forming ion-pairs e.g. TOAH+P− (P− is the acylpyrazolone anion) and depending on the experimental conditions the synergistic agents can be the amine salt or the ion-pair. Analogues ideas have been proposed by Umetani et al.94 for the synergistic extraction of Zn and Cd with mixtures of HTTA and Aliquat 336. However, the authors94 attributed the synergism in the extraction of Zn and Cd with 4-benzoyl-3-methyl-1-phenyl-5-pyrazolone (HPMBP) and capriquat (trioctylmethylammonium chloride, QCl) to the formation of ion-pairs Q+(PMBP)− obtained as a result of the interaction between the extractants. Formation of species ZnP2(Q+PMBP−) and CdP2(Q+PMBP−) has been established. The authors have assumed that the higher acidity of HPMBP, in comparison with HTTA, facilitate the interaction with the capriquat but when the concentration of capriquat has become close to that of HPMBP, they have observed destruction of synergism for both metals. On the other hand, Saeed et al.95 have not validated the formation of anionic species when Eu(III) was extracted with 1-phenyl-3-methyl-4-trifluoroacetylpyrazol-5-one (HPMTFP) and tribenzylamine (TBnA) mixtures, but adducts Eu(PMTFP)3·TBnA at lower and Eu(PMTFP)3·2TBnA at higher concentration of TBnA were detected, (0.01 M). No information for TBnAHClO4 creation in HClO4 medium or observed possible interaction has been reported. The effect of most commonly used anions as their sodium or potassium salt or cations (10 mg ml−1) have also been examined on the Eu(III) extraction.95 The data show that among the various ions tested fluoride, phosphate, EDTA, Cu(II) and Ti(IV) have drastically reduced Eu(III) extraction yield.
As a whole, the possible interaction between the chelating extractant and the quaternary ammonium salt can be represented by the equation: HL(o) + QA(o) ⇌ QL(o) + H+(aq) + A−(aq), where L− is the anion of the chelating compound, A− is anion of the amine salt and “o” and “aq” denote organic and aqueous phase respectively. The experimental data obtained in 2001 by our group, for the interaction between thenoyltrifluoroacetone and the quaternary ammonium salt, Aliquat 336 are shown in Fig. 1 and 2.96 It is possible to conclude that the interaction between the chelating extractants 4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one (HP) or HTTA with chloride and perchlorate forms of the quaternary ammonium salt depends strongly on the salt anion. The calculated values of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kexc are given in Table 2. It is seen that the interaction between HP or HTTA with QClO4 is negligibly small even at rather high pH values (at high pH, lower H+ concentration, the equilibrium described with the above equation would be shifted to the side of the ion-pair formation) (Fig. 1, curve 3). The concentrations of the ion-pair do not differ significantly for HP–QClO4 and HTTA–QClO4 e.g. the concentration of QP in the system 3 × 10−2 M HP and 3 × 10−2 M QClO4 at pH = 5.90 is around 1.5 × 10−5 M and the concentration of QTTA at pH = 5.96 in the binary system 5 × 10−2 M HTTA and 5 × 10−3 M QClO4 is 1.6 × 10−5 M. After the screening, the interaction between the chelating extractants and QCl can be evaluated as larger but the concentration of the ion-pair is still low. The maximal concentration of QP obtained is approximately 2.25 × 10−3 M at initial concentration of both HP and QCl equal to 3 × 10−2 M. While, the maximal concentration of QTTA is 4 × 10−3 M approximately at [HTTA]i = 5 × 10−2 M and [QCl]i = 3 × 10−2 M.
Kexc are given in Table 2. It is seen that the interaction between HP or HTTA with QClO4 is negligibly small even at rather high pH values (at high pH, lower H+ concentration, the equilibrium described with the above equation would be shifted to the side of the ion-pair formation) (Fig. 1, curve 3). The concentrations of the ion-pair do not differ significantly for HP–QClO4 and HTTA–QClO4 e.g. the concentration of QP in the system 3 × 10−2 M HP and 3 × 10−2 M QClO4 at pH = 5.90 is around 1.5 × 10−5 M and the concentration of QTTA at pH = 5.96 in the binary system 5 × 10−2 M HTTA and 5 × 10−3 M QClO4 is 1.6 × 10−5 M. After the screening, the interaction between the chelating extractants and QCl can be evaluated as larger but the concentration of the ion-pair is still low. The maximal concentration of QP obtained is approximately 2.25 × 10−3 M at initial concentration of both HP and QCl equal to 3 × 10−2 M. While, the maximal concentration of QTTA is 4 × 10−3 M approximately at [HTTA]i = 5 × 10−2 M and [QCl]i = 3 × 10−2 M.
 | ||
| Fig. 1 Effect of the initial value of pH (pHi) on the concentration of the ion-pair QTTA (CQTTA): (1) 5 × 10−2 M HTTA + 3 × 10−2 M QCl; (2) 5 × 10−2 M HTTA + 5 × 10−3 M QCl; (3) 5 × 10−2 M HTTA + 5 × 10−3 M QClO4.96 © 2001 J. of UCTM. | ||
 | ||
| Fig. 2 Effect of concentration of QCl (CQCl) on the concentration of the ion-pair QTTA (CQTTA) at [HTTA] = 5 × 10−2 M and (1) pHi = 2.41; (2) pHi = 3.68; (3) pHi = 4.65.96 © 2001 J. of UCTM. | ||
| Extractants | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kexc Kexc |
|---|---|
| HP + QCl | −2.68 ± 0.03 |
| HP + QClO4 | −6.80 ± 0.06 |
| HTTA + QCl | −2.52 ± 0.03 |
| HTTA + QClO4 | −6.63 ± 0.05 |
The plots of CQTTA vs. CQCl, Fig. 2, show that the tendency of increasing interaction between the extractants with increasing pH values is preserved, as usual.
Surprisingly, the values of Kexc for HP–QA and HTTA–QA systems are practically equal despite the great difference of the pKa values for HP and HTTA, Table 2. This fact could be explained with the important role of the quaternary ammonium salt, because the formation of the ion-pair depends on the breaking of the bond between the cation and the anion of the salt. It is known that the bond energy increases in the order chloride < nitrate < perchlorate and because of that the values of Kexc decrease in that order, Table 2. The breaking of the bond in QClO4 is difficult and the interaction between the extractants in systems with its participation is difficult too. The diluents effect on the interaction was investigated as well by our research group, Fig. 3.97 It was found that the values of the equilibrium constant Kexc increased in the order CHCl3 < C6H6 < CCl4 < C6H12 for both HTTA (HP)–QA combinations, Table 3.
 | ||
| Fig. 3 Effect of the initial value of pH (pHi) on the concentration of the ion-pair QP: 3 × 10−2 M HP + 3 × 10−2 M QCl (curves 1–4); 3 × 10−2 M HP + 5 × 10−3 M QCl (curves 1*–4*); diluents: (1–1*)-C6H12; (2–2*)-CCl4; (3–3*)-C6H6; (4–4*)-CHCl3.97 © 2003 J. of UCTM. | ||
| Diluents | HTTA | HP | ||
|---|---|---|---|---|
| QCl | QClO4 | QCl | QClO4 | |
| C6H12 | −2.36 ± 0.03 | −6.37 ± 0.07 | −2.23 ± 0.02 | −5.72 ± 0.06 |
| CCl4 | −2.45 ± 0.04 | −6.52 ± 0.09 | −2.42 ± 0.03 | −6.35 ± 0.07 |
| CHCl3 | −3.91 ± 0.05 | −7.58 ± 0.09 | −3.83 ± 0.02 | −7.02 ± 0.07 |
It is a known fact that diluents with high solvation ability stabilize the polar amine salts.98 Therefore, the formation of the ion-pair is hindered in higher extent when such diluents are used and because of that the values of Kexc are lower. In accordance to the abovementioned, the values of Kexc decreased in the order: cylohexane < xylene < carbon tetrachloride < toluene < benzene < chloroform.97 For all investigated diluents the change of the quaternary ammonium salt anion caused a decrease of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kexc value of about 3 orders of magnitude.
Kexc value of about 3 orders of magnitude.
The influence of the interaction between HP and Aliquat 336 in chloride (QCl) and perchlorate form in C6H6 on the synergistic extraction of Pr(III) has been studied by Dukov et al. in detail, Fig. 4.99 It was found that at the applied experimental conditions the concentration of the ion-pair QP obtained as a result of the extractants interaction is low and that the synergistic agent is the quaternary ammonium salt itself. The change of the anion (Cl− to ClO4−) causes a significant decrease of the equilibrium constant as the formation of the extracted complex Q[PrP4] is connected with the breaking of the bond between the cation and the anion of the salt:
| Pr3+(aq) + 4HP(o) + QA(o) ⇌ Q[PrP4](o) + 4H+(aq) + A−(aq) | (1) |
 | ||
Fig. 4 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DP,Q vs. log[HP] for Pr(III) extraction with mixtures of HP and QA. [QA] = 5 × 10−3 M. (1) QCl, pH = 1.90; (2) QClO4, pH = 2.90; open cicrcles-without pre-equilibration of HP and QA; closed circles-pre-equilibration of HP and QA.99 © 2001 Marcel Dekker, Inc. DP,Q vs. log[HP] for Pr(III) extraction with mixtures of HP and QA. [QA] = 5 × 10−3 M. (1) QCl, pH = 1.90; (2) QClO4, pH = 2.90; open cicrcles-without pre-equilibration of HP and QA; closed circles-pre-equilibration of HP and QA.99 © 2001 Marcel Dekker, Inc. | ||
The experiments for the extraction of Pr(III) without and with pre-equilibration of the extractants solution (extractant's concentration was appropriate for the metal extraction) have shown that there is no difference of the distribution ratios of this 4f metal in both cases. The previous investigation in 1981, of the ternary synergistic system Pr–HTTA–Aliquat 336 (QCl)–TOAHCl has shown similar results, Fig. 5.100 The weaker synergistic agent (TOAHCl) has not exerted detectable influence on the extraction process. It has been established that at [HTTA] = 8 × 10−2 M, [QCl] = 3.6 × 10−4 M and [TOAHCl] = 2.5 × 10−3 to 7.5 × 10−3 M ligand concentrations, the complex Q[Pr(TTA)4] is the one of real interest.
 | ||
Fig. 5 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DI vs. pH at a constant [HTTA] = 8 × 10−2 M and [QCl] = 3.6 × 10−4 M; [TOA] = 2.5 × 10−3 M (Δ), 5 × 10−3 M (○), 7.5 × 10−3 M (●), 10−2 M (□).100 © 1981 Elsevier. DI vs. pH at a constant [HTTA] = 8 × 10−2 M and [QCl] = 3.6 × 10−4 M; [TOA] = 2.5 × 10−3 M (Δ), 5 × 10−3 M (○), 7.5 × 10−3 M (●), 10−2 M (□).100 © 1981 Elsevier. | ||
In a series of papers Brunette and co-researchers88–93,101–104 have investigated the synergistic solvent extraction of divalent metals (Co, Ni, Zn, Cd) with various acylpyrazolones (mainly HPMBP) and primary, ternary and quaternary ammonium salts (dodecylamine (DOA), tribenzylamine (TBnA), trioctylamine (TOA), Aliquat 336 in sulfate, nitrate, chloride and perchlorate forms). Formation of complexes AmH+[Mn+Pn+1]− and Q+[Mn+Pn+1]− (P is the anion of the respective acylpyrazolone) have been established in all studied systems except for TBnA which has been poorly protonized at the experimental conditions.90 It has been found that in the organic phase TBnA is in a neutral form and as consequence no synergism has been observed in this particular case. The Strasbourg scientific group have concluded that the formation of anionic complexes can be formed through two different but thermodynamically equivalent ways viz. when the synergistic agent is an ion-pair AmH+P− or Q+P− or the synergistic agents are the ammonium salts AmHA or QA (A = SO4−, Cl−, NO3−, ClO4−).101 According to the authors the interaction between the extractants has been predominant in the systems involving sulfate ions, whereas ammonium salts are predominating in systems involving nitrate and perchlorate media.90 The authors have established that the quaternary ammonium salt is a better synergist than ternary and primary ammonium salts in sulfate or chloride media but this difference is strongly reduced in nitrate and perchlorate media, in which the ratio [AmHA(QA)/[AmH+P− (Q+P−)] has been found to be high.104
Brunette and co-workers88,89 have found that the interaction between acylpyrazolones and ternary or quaternary ammonium salts in sulphate, chlorine or nitrate forms is significant and then the synergist is the ion-pair Q+·P− or AmH+·P− but the interaction with perchlorate form of the salts is negligible and the synergist is the salt. The authors have noted that in both cases the composition of the extracted species is Q+[Mn+Pn+1] as noted above and that the two mechanism are thermodynamically equivalent. However, these ideas cannot be accepted without doubts. If the synergist was the compound Q+·TTA− the equilibrium: HTTA + QA ⇌ Q+·TTA− + H+ + A− should be shifted to the right because the compound Q+·TTA− could be consumed for the formation of the anionic metallic complexes. On the other hand, if the above-mentioned suggestion was true, the salt anion should not cause any influence on the extraction mechanism. As the experimental data show the synergistic extraction is strongly dependent on the quaternary ammonium salt, it is logical to be accepted that the synergist is the ammonium salt and the anionic mixed complexes will be formed in accordance with eqn (1). In such a case, according to the Le Chatelier's principle the equilibrium connected with the ion-pairs formation will be shifted to the left and the ion-pairs (as far as they are formed) will be destroyed. In the opposite case antagonism (destruction of synergism) will occur. Such an effect was reported by Zhang et al.105,106 for palladium(II) extraction. The results presented in Fig. 6 indicates that there is obviously an antagonistic effect in the HPMTP + TOA (1-phenyl-3-methyl-4-trifluoroacetylpyrazolone-5-one, tri-n-octylamine) chloroform system, because the distribution ratio (D12) decreases by increasing HPMTP concentration and no maximum value appears.
 | ||
Fig. 6 Dependence of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D12 on the total concentration of TOA. ([Pd(II)](aq) = 3.52 × 10−4 M, pH = 1.96, μ = 0.1 M; [HPMTP](o) = ×102 M: (1)-1025, (2)-1.63, (3)-1.96, (4)-2.32, (5)-2.68, (6)-3.01).105 © 2004 Springer Science + Business Media, Inc. D12 on the total concentration of TOA. ([Pd(II)](aq) = 3.52 × 10−4 M, pH = 1.96, μ = 0.1 M; [HPMTP](o) = ×102 M: (1)-1025, (2)-1.63, (3)-1.96, (4)-2.32, (5)-2.68, (6)-3.01).105 © 2004 Springer Science + Business Media, Inc. | ||
The antagonistic effect is therefore explained by a decrease of the available chelating reagent concentration in the organic phase due to the association reaction, which can be expresses as:
| HPMTP(o) + jTOA(o) ⇌ HPMTP·jTOA(o). |
The equilibrium constant βj is calculated to be 2.86 ± 0.05. In addition, based on the extractants structures, it is possible that N atom in the TOA molecule associates with the H atom in the enol-form of the HPMTP to form a hydrogen bonding as illustrated in Fig. 7.
A significant antagonistic extraction effect was observed in the extraction system HPMPP (1-phenyl-3-methyl-4-propionylpyrazolone-5-one) and tertiary amine R1R2R3N (R1, R2 and R3 are octyl, nonyl and decyl groups, respectively) due to the formation of the species HPMPP·NR1R2R3 (calculated equilibrium constant 2.2 ± 0.1), Fig. 8.
It was demonstrated by Zhang that no maximum value appears in the extraction curve, Fig. 9, but a minimum value exists, indicating that the extraction of Pd(II) by HPMPP takes place in the organic phase to form the chelates Pd(PMPP)2 without the tertiary amine participation.
 | ||
| Fig. 9 Relationship between D12 and χHPMPP. [Pd(II) = 4.52 × 10−4 M, [HPMPP] + [R1R2R3N](o) = 4.21 × 10−2, pH = 2.30.106 © 2001 Marcel Dekker, Inc. | ||
Deptula and Mine107 have reported a synergistic effect in uranium extraction from sulphuric acid solution into CCl4 by di-2-ethylhexylphosphoric acid (HDEHP) plus tri-n-octylamine (TOA) suggesting the formation of the following compound:
The authors have established an antagonistic effect due to the interaction between TOA and HDEHP. According to the authors occurrence of the synergism or antagonism depends on the sulphuric acid concentration in the aqueous phase (0.1–2 M H2SO4).
It was found out by Fleitlikh et al.108 that during the extraction of In(III) a strong antagonistic effect takes place caused by the interaction between D2EHPA (di-(2-ethylhexyl)phosphoric) and octanoic (HA) acid molecules i.e. two acidic reagents at the expense of formation of intermolecular hydrogen bonds. As suggested by the authors this effect seems to be further related to simplification of indium re-extraction from the organic phase, which is necessary for the development of technology for indium recovery from the solutions of zinc industrial production.
The experimental data96,99,100 show that when synergism occurs the coordination of the extractants to the metal ion have to be stronger than the bond in the ion pair (if any) and then the formation of mixed complexes would cause destruction of the ion-pairs because the equilibrium describing their formation should be shifted to the left. In the opposite case antisynergistic effect should be observed. Since at the experimental conditions of the lanthanoid(III) extraction described, synergism has been found for all studied systems, it could be suggested that the interaction between the chelating extractants and primary, secondary, tertiary and quaternary ammonium salts does not influence significantly on the synergistic process.96,99,100
3 Interaction between acidic (chelating) agents and phosphorus-containing neutral ligands
The possible interactions between 4-(4-fluorobenzoyl)-3-methyl-1-phenyl-pyrazol-5-one (HL1) and a series of phosphine oxides (TOPO, tributylphosphine oxide (TBPO), triphenylphosphine oxide (TPPO)) and tributylphosphate (TBP) have been studied by proton, carbon and phosphorus NMR spectra in benzene-d6 solutions by Petrova et al.109 At this point, it has been concluded that trialkylphosphine oxides (TOPO and TBPO) form strong H-bonds with HL in the organic phase bases on two main observations. First, significant shifting of HL signals has been detected (Table 4) and it was found that the effect is strongly dependent on the extractants' ratio; the values of HL proton and carbon and S phosphorus signals shifting increase with the decrease of the HL and S part, respectively. Second, slow exchange between two sites in HL proton spectra has been detected upon mixing indicating that the strong intramolecular OH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O H-bonding in HL1 is destroyed due to bonding with P
O H-bonding in HL1 is destroyed due to bonding with P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the synergist. Similar but much less significant effects have been observed in arylphosphine oxide and phosphate spectra indicating very weak interactions with HL1.
O of the synergist. Similar but much less significant effects have been observed in arylphosphine oxide and phosphate spectra indicating very weak interactions with HL1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixtures in benzene-d6
2 mixtures in benzene-d6
| Signal | HL1/S | HL1 + TOPO | HL1 + TBPO | HL1 + TPPO | HL1 + TBP | ||||
|---|---|---|---|---|---|---|---|---|---|
| δ | δ | Δδ | δ | Δδ | δ | Δδ | δ | Δδ | |
| CH3 | 1.823 | 2.237 | +0.414 | 2.211 | +0.388 | 1.943 | +0.120 | 1.846 | +0.023 |
| C5 | 162.52 | 158.22 | −4.30 | 158.98 | −3.54 | 161.53 | −0.99 | 162.37 | −0.15 |
| C4 | 147.33 | 148.99 | +1.66 | 148.87 | +1.54 | 147.77 | +0.44 | 147.41 | +0.08 |
| C3 | 103.91 | 105.04 | +1.13 | 104.96 | +1.05 | 104.19 | +0.28 | 103.96 | +0.05 |
P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
42.72 | 46.25 | +3.53 | ||||||
P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
44.02 | 46.59 | +2.57 | ||||||
P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
25.77 | 26.35 | +0.58 | ||||||
P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
1.08 | 0.98 | −0.10 | ||||||
By considering the interaction reaction between HTTA and TOPO in cycloxehane Favaro and Atalla have identified the species Ln(TTA)3·TOPO and Ln(TTA)3·2TOPO for La and Yb.110 The bahaviour of the system changes when TOPO concentration is higher than 0.15 M as a result of a gradual transformation of the enolic form of HTTA into the keto-hydrated and its consecutive interaction with TOPO. The distribution of La and Yb as a function of the HTTA concentration induces the slopes of the straight lines lower than the expected value of 3 (2.1 for La and 2.7 for Yb). This disagreement was assumed as resulting from HTTA–TOPO interaction.
D. Olieslager and Sannen have been reported the extraction data of Eu(III) and Tm(III) from aqueous HClO4/NaClO4 solutions with benzoylacetone (HBTA)/tri-n-butylphosphate (TBP) in benzene.111 At low concentration (<0.02M) the synergism was explained by the formation of complexes of Ln-β-diketonate with one or two mole of the synergistic agent while at high concentration of TBP a destruction of synergism was observed due to the formation of hydrated species:
| nHBTA(o) + mTBP(o) + rH2O(o) ⇌ (HBTA)n(TBP)m(H2O)r(o) |
Comparing the effect of the mixtures HTTA plus TBP and bis(2-ethylhexyl)phosphoric acid (HDEHP) plus TBP in the lanthanoids extraction, Baes attributed the differences observed in their behaviour to two major factors:112 (i) the complexes formed between M(A2H)z and TBP are much less stable than those established between M(TTA)z and TBP, where A2H is a dimer of HDEHP and z is the metal charge; (ii) the interaction between MDEHP and TBP is much stronger than that between HTTA and TBP, because the hydrogen bond among the two phosphoryl oxygens is stronger than that amid a phoshoryl group (from TBP) and a carboxylic HTTA group.
The results obtained suggest that the association between the acidic and the neutral components is comparatively stronger, since it may take place through the monomerization of the dimeric alkylphosphoric acid, according to the reaction:
| (HX)2 + 2S ⇌ 2HX·S or HX + S ↔ HX·S |
| K22 = [HX·S]2[(HX)2]−1[S]−2 and K11 = [HX·S][HX]−1[S]−1. |
In either case, the reaction involves the rupture of two hydrogen bonds in the de-dimerization of the dimeric acidic phosphorus ester, and the formation of two new ones between two pairs of HX and S molecules. Thus, in what concerns the stability of the dimer, affected on the diluent employed, that of the mixed adduct will also depend on the diluents nature. As a final point, this has been shown to be true in a variety of systems, involving various acidic and neutral phosphorus reagents, Table 5.21 Furthermore, the extent of arrangement of mixed associates depends on the basicity of S and as consequence, a higher stability constant will be expected with more basic neutral ligands.
| Acidic component | Neutral component | Diluent | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K11 K11 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K22 K22 |
|---|---|---|---|---|
| a HDBP-di-n-butylphosphorc acid; EPMP-diethylpolystyrene methylenephosphonate resin; DEHPD-di-(2-ethylhexyl)phosphoric acid; DIPE-diisopropyl ether; MIBK-methylisobutyl ketone. | ||||
| HDBP | TBP | Kerosine | 2.83 | −0.12 |
| HDBP | TBP | Hexane | 2.94 | −0.78 |
| HDBP | TBP | Chloroform | 1.60 | −1.41 |
| HDBP | TBP | CCl4 | 2.65 | −1.07 |
| HDBP | TBP | TBP | 2.84 | 5.80 |
| HDBP | TOPO | Hexane | 4.88 | 3.10 |
| HDBP | TOPO | CCl4 | 4.36 | 2.35 |
| HDBP | MIBK | MIBK | 1.86 | 2.53 |
| HDBP | DIPE | DIPE | 1.06 | −0.17 |
| HDBP | Chloroform | Chloroform | 0.53 | −3.55 |
| HDBP | Nitrobenzene | Nitrobenzene | 0.25 | −3.05 |
| HDBP | EPMP | Benzene | −0.92 | |
| HDEHP | TOPO | n-Octane | 4.28 | 4.09 |
Moreover, it has been suggested that the association between the non-identical molecules of HX and S may not necessarily involve the monomerization of the dimeric alkylphosphoric acid. It may be possible that the adduct formation proceeds, at least partially, according to the reaction:
| (HX)2 + S ⇌ (HX)2·S, K21 = [(HX)2S][(HX)2]−1[S]−1. |
The interaction is assumed to take place again through hydrogen bonding between P–OH and the phosphoryl oxygen of the neutral ligand, but the adduct is composed upon the rupture of one hydrogen bond in the dimeric (HX)2:
| (RO)2(O)P–OH⋯OP(OR)2–OH⋯O ← PR3 |
Particularly notable is that the stability increases in the order of increasing base strength of S, which increases adequate with the same order of diluents, Table 6.
| Acidic component | Neutral component | Diluent | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K21 K21 |
|---|---|---|---|
| a HEH2EHP-2-ethylhexyl-2-ethylhexylphosphoric acid; BDBP-n-butyl di-n-butylphosphinate; HDMBPP-di-(1,1,3,3-tetramethylbutyl)phenylphosphoric acid. | |||
| HEH2EHP | D2EH2EHP | Cyclohexane | 1.30 |
| HEH2EHP | DBBP | Cyclohexane | 1.30 |
| HEH2EHP | TOPO | Cyclohexane | 2.14 |
| HDEHP | D2EH2EHP | Cyclohexane | 1.30 |
| HDEHP | DBBP | Cyclohexane | 1.30 |
| HDEHP | TOPO | Cyclohexane | 2.30 |
| HDMBPP | D2EH2EHP | Cyclohexane | 2.60 |
| HDMBPP | DBBP | Cyclohexane | 2.74 |
| HDEHP | TBPO | Kerosene | 1.52 |
| HDEHP | BDBP | Kerosene | 1.25 |
| HDEHP | DBBP | Kerosene | 0.78 |
| HDEHP | TBP | Kerosene | 0.60 |
| HDEHP | TBP | Hexane | 0.40 |
| HDEHP | TBP | CCl4 | 0.04 |
| HDEHP | TBP | Benzene | 0.00 |
| HDBP | TBP | Hexane | 1.27 |
| HDBP | TBP | CCl4 | 0.22 |
| HDBP | TOPO | Hexane | 2.60 |
Nevertheless, it should be also remarked as shown by UV and 31P NMR spectroscopies by Goetz-Grandmont et al.,113 that interaction of TOPO with HPBI (3-phenyl-4-benzoyl-isoxazol-5-one) is stronger than including 4-acyl-5-hydroxypyrazolones (acyl: lauroyl(HPMLP), benzoyl(PMBP), thenoyl(HPMTP)). The most likely tautomeric form for HPBI in the H-bounded TOPO·HPBI moiety is the diketo-enamine. Molecular modelling applications show that the R3PO⋯H–NHPBI hydrogen bond is 0.15 shorter that the R3PO⋯H–OHPBI one, when comparing two isomeric forms of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TOPO·HPBI complex. The hypochromic effect characterized by εL/εTL on the high wavelength UV band, Fig. 10, was observed to be stronger in wet toluene (1.22) than in wet chloroform (1.14). For the reaction:
1 TOPO·HPBI complex. The hypochromic effect characterized by εL/εTL on the high wavelength UV band, Fig. 10, was observed to be stronger in wet toluene (1.22) than in wet chloroform (1.14). For the reaction:
| HL + TOPO ⇌ TOPO·HL, |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kint = 1.05, 1.08, 1.22 and 1.85 respectively for HPMLP, HPMBP, HPMTP, HBPBI in toluene were obtained.
Kint = 1.05, 1.08, 1.22 and 1.85 respectively for HPMLP, HPMBP, HPMTP, HBPBI in toluene were obtained.
 | ||
| Fig. 10 UV spectra of solutions 1 × 10−3 M HPBI + y × 10−3 M TOPO in wet toluene. From top to bottom: y = 0, 1, 5, 10, 25, 50, 95. Solvent cut-off at 280 nm.113 © 1996 Taylor & Francis. | ||
The synergistic extraction of samarium into toluene using two different main extractants (dibutylmonothiophosphoric acid (DBTPA) and dibutylphosphoric acid (DBPA)) and three organic phosphine oxides as synergists (TBPO, dibutylphosphate (DBOBPO) and tri-n-butylphosphate (TBOPO)) was examined by Kondo et al.114 The calculated values of the association constants (KS,HR) between the ligands are listed in Table 7.
| Synergist | |||
|---|---|---|---|
| TBPO | DBOBPO | TBOPO | |
| DBTPA | 3.77 | 2.98 × 10−1 | 1.08 × 10−1 |
| DBPA | 1.37 | 5.27 × 10−1 | 9.30 × 10−2 |
As expected, the magnitude of KS,HR for the synergists was in the order, TBPO > DBOBPO > TBOPO. The authors explained the antagonistic effect observed in the mixed system DBPA–TBPO as a result of ligands association and the decreases of the free main extractant concentration.
Reddy et al.115 have used dihexyl-N,N-diethylcarbamoylmethylphosphonate (CMP) as a neutral donor in combination with HP for the extraction of trivalent 4f- and 5f-ions (La, Eu, Lu and Am) into xylene from 0.01 mol dm−3 chloroacetate buffer solutions. About 3-fold to 20-fold enhancement in the extraction of these metal ions has been observed upon addition of the synergist CMP. The authors have determined the constant log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KI to be 0.24 ± 0.01 by non-linear regression analysis of the side reaction between the two ligands written as HP(o) + CMP(o) ⇌ HP·CMP(o), but no supplementary comments have been reported about its influence inasmuch as better extraction efficiency and selectivity with these mixed system were obtained.
KI to be 0.24 ± 0.01 by non-linear regression analysis of the side reaction between the two ligands written as HP(o) + CMP(o) ⇌ HP·CMP(o), but no supplementary comments have been reported about its influence inasmuch as better extraction efficiency and selectivity with these mixed system were obtained.
In order to reach a better understanding of the synergistic extraction of lanthanoids, the NMR spectra of selected β-dicarbonyl compounds, namely 3-methyl-4-(4-methylbenzoyl)-1-phenyl-pyrazol-5-one (HL2), and thenoyltrifluoroacetone (HTTA), and N,N-diisobutyl-2-(octylphenylphosphoryl)acetamide (CMPO) have been recorded in CDCl3 solutions and compared with those of their mixtures in different molar ratios (Fig. 11) with a view to identify some distinct changes.116 The CMPO compound leads to certain benefits in f-ions extraction due to its particular properties.
Mention should be made here, the proton and carbon spectra have displayed negligible shifting of the signals upon mixing. Even the highly sensitive to structural and environmental changes phosphorus resonances have shown insignificant chemical shift differences (Table 8). Based on these observations it has been concluded that no interactions occur in chloroform solutions, independently on HL2/CMPO or HTTA/CMPO proportions. The latter has been confirmed by NOESY experiments where only intramolecular interactions for both components of the systems have been registered, while no intermolecular cross peaks were detected.
| HL | Δδ, HL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMPO 1 CMPO 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Δδ, HL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMPO 1 CMPO 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Δδ, HL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMPO 2 CMPO 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|---|---|---|---|
| HL2 | +0.05 | +0.06 | +0.11 |
| HTTA | +0.07 | +0.09 | +0.15 |
Another worthy example is at low acidity, two different behaviour depending on the metal concentration were observed by Muller et al.117 for lanthanoids extraction by a mixture of a malonamide (DMDOHEMA) and a dialkylphosphoric acid (HDEHP), (HNO3, hydrogenated tetrapropylene), Fig. 12.
A synergistic effect for macro-concentrations and an antagonistic effect for tracer levels due to the adduct formation:
| aDMMDOHEMA(o) + bHDEHP(o) ⇌ (DMMDOHEMA)a·(HDEHP)b(o) |
FT-IR spectroscopy was used by the authors to identify a possible interaction that requires some additional comments were ascertained, Fig. 13.
 | ||
| Fig. 13 FT-IR spectra of 0.6 M DMDOHEMA (blue), 0.3 M HDEHP (red) and 0.6 M DMDOHEMA + 0.3 M HDEHP (black) in TPH.117 © 2016 Taylor & Francis. | ||
No change was observed on the mixture IR spectra indicating that the strength of the interaction would be too weak to induce some changes. Furthermore, the strength of an HDEHP-neutral extractant adduct depends on the basicity of the neutral ligand: the more basic the compound is, the stronger will be the interaction. So, the strength of the extractant varies in the following order: TOPO > CMPO > malonamides.118 Then, the DMDOHEMA–HDEHP interaction would be weaker than known interactions in HDEHP–TOPO119 or HDEHP–CMPO system.120
With the goal of promoting effective and more environmentally friendly mixed extraction systems, Atanassova et al. employed solutions of imidazolium based ILs. As a decisive approach, our group studied the interactions between a series of differently substituted 4-aroyl-3-methyl-1-phenyl-pyrazol-5-ones (HL) and phosphorylated at the narrow rim calix[4]arenes possessing variable number of substituents and length of the bridged chain in the role of synergistic agents (Fig. 14) by NMR spectra.121,122
It has been shown that all pyrazolones in chloroform solutions exist as pure enol forms with strong intramolecular H-bonding and that calixarene have not caused conformational changes upon mixing. It has been concluded that no substantial interactions occurred between tetrasubstituted calixarene S1 and 4-methyl-,121 4-fluoro-121 and 4-biphenyl122 pyrazolones (HL1–HL3) as only slight shifting of phosphorus signals have been detected, up to 0.24 ppm.
Contrary, the spectra of partially substituted calixarenes S2 and S3 have shown shifting of the signals when mixing with 4-(trifluoromethyl)benzoyl pyrazolone (HL4).123 The most significant changes in carbon and proton resonances have been detected for pyrazolone methyl-3 and Cq-3 and for calixarene methyl and methylene groups neighboring to phosphorus. Additionally, broad signals for some HL4 protons were registered due to hindered rotation around C4–CO bond. It was concluded that the intramolecular H-bond in HL4 is partially destroyed due to interaction with calixarene P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and that the interactions are localized on the pyrazolone hydroxyl group, while the carbonyl function is not involved. On the other hand, the phosphorus spectra have displayed the first suggestion as substantial shifting downfield has detected upon mixing (Fig. 15). It has observed that the effect is dependent on the S/HL4 proportion and that both phosphorus resonances of S2 are not equally shifted; +0.58 ppm for the double signal vs. +0.79 ppm for the middle group, from S2 to S2
O groups and that the interactions are localized on the pyrazolone hydroxyl group, while the carbonyl function is not involved. On the other hand, the phosphorus spectra have displayed the first suggestion as substantial shifting downfield has detected upon mixing (Fig. 15). It has observed that the effect is dependent on the S/HL4 proportion and that both phosphorus resonances of S2 are not equally shifted; +0.58 ppm for the double signal vs. +0.79 ppm for the middle group, from S2 to S2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HL4 1
HL4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.
3.
 | ||
Fig. 15 31P NMR spectra of S2 and S2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HL4 mixtures (down) and S3 and S3 HL4 mixtures (down) and S3 and S3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HL4 mixtures (up) in CDCl3; S (brown), S HL4 mixtures (up) in CDCl3; S (brown), S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HL4 1 HL4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (green), S 1 (green), S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HL4 1 HL4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (violet). 3 (violet). | ||
Based on the comparison between the observations in different acidic/neutral systems previously presented, it has been suggested that: (i) the free OH groups of calixarenes S2 and S3 are involved in the interactions with pyrazolone chelating arm; and (ii) that tetrasubstituted calixarene S1 behaves similarly to TBP most probably due to the electronic effect of the oxygen or/and steric hindrance.
Liquid–liquid extraction of cesium (137Cs isotope) with 18-crown-6 in nitrobenzene was diminished in the presence of various phosphine oxides, TOPO, TBPO and TPPO.124 Formation of an electron donor–acceptor complex between the crown ether and phosphine oxides is assumed, making the crown ether less available for cesium extraction. The formation constants of the complexes were determined by UV-Vis spectrophotometry, utilizing Benesi–Hildebrand equation.125–131 This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground to the excited state.132
Particularly relevant are the works focused on the apparent presence of antagonistic effects in some of the systems investigated by McAlister et al.133 for extraction of alkaline (Ca2+, Sr2+, Ba2+ and Ra2+) and actinoid (Am3+, UO22+, Th4+) cations by mixtures of di(2-ethylhexyl)alkylendiphosphoric acids and various neutral ligands (dicyclohexano-18-crown-6 (DCH18C6), 21-crown-7 (21C7), dicyclohexano-21-crown-7 (DCH21C7), TBP, TOPO, diamyl amylphosphonate (DA[AP])) in xylene arising to two sources: an interaction between the two extractants or partial dissolution of the crown ether in the aqueous phase, leading to aqueous complex formation. To distinguish between these two possibilities, IR spectroscopy was performed on mixtures of DCH18C6 and H2DEH[MDP] (methylen) in toluene indicating an absence of interaction supported by vapor pressure osmometry survey. But a significant antagonistic effect brought by the neutral organophosphorus compounds for the Am(III) extraction is particularly evident as the neutral molecule becomes more basic. Table 9 reports the lowest neutral to acidic extractant concentration ratios at which shifts in the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequencies were observed. The examination of the infrared spectra of H2DEH[MDP] and TBP, both alone and as mixtures in CCl4, indicates that little interaction takes place between them even when the diluent is completely replaced with TBP. Analogous studies for H2DEH[EDP] (ethylene) suggest that, in all cases, interactions occur at neutral ester-to-acid ratios exceeding 1
O stretching frequencies were observed. The examination of the infrared spectra of H2DEH[MDP] and TBP, both alone and as mixtures in CCl4, indicates that little interaction takes place between them even when the diluent is completely replaced with TBP. Analogous studies for H2DEH[EDP] (ethylene) suggest that, in all cases, interactions occur at neutral ester-to-acid ratios exceeding 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
| Acidic ligand | Neutral | Extractants | |
|---|---|---|---|
| TBP | DA[AP] | TEHPO | |
| a TEHPO-a liquid analog of TOPO employed to facilitate solution preparation. | |||
| H2DEH[MDP] | Little interaction | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| H2DEH[EDP] | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| H2DEH[BuDP] (butylene) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Thus, neutral organophosphorus esters do interact with diphosphonic acids, with strength of the interaction increasing with the basicity of the phosphoryl group of the neutral compound. The presence of these molecules in mixtures would be expected to provoked a disruption of the aggregation of the diphosphonic acids and, more importantly, to a reduction in their effectiveness as extractants. More noteworthy, however, is that the length of the alkylene bridge separating the two phosphorus atoms of the acid is the factor ultimately governing the magnitude of synergistic effects in extraction systems based on di(2-ethylhexyl)alkylene diphosphoric acids.
4 Conclusions
The debates about the interaction between the extractants in the mixed systems have been arisen almost immediately with the appearance of the first studies devoted to the synergistic solvent extraction of metal ions. At this point based on this literature review it may be clear that the possible interaction of ligands during extraction of metallic species by mixtures of two or more compounds is not a reaction with foremost importance when synergism occurs. It is apparent from the foregoing discussion that the statement, inter-ligand reaction always leads to destruction of synergism if occurs in the organic media and plays a crucial role, should not be accepted without strong reservations according to some authors. Although, in the daybreak of synergism in the liquid–liquid metal recovery (1959–1962), the researchers have stated that this reaction comes along to adverse effects either in the degradation of the extraction efficiency or in the ligand irreversible loss due to an association products formed among the two coordination molecules.8,19,26 Furthermore, the examination of the publications devoted to the metal ions solvent extraction with mixtures of chelating extractants and various alkylammonium salts, it has shown that there are different opinions about the role of the ion-pairs formed by interaction between the ligands during the extraction proceeding. Some authors have accepted that chelating extractants (weak acids) and alkylamines (weak bases) are able to interact with each other during the extraction process and that the synergistic enhancement is due to the species result of such interaction but other scientists have found that this interaction have no contribution in metal extraction efficiency. There is widespread opinion that in the synergistic region of the reactions the chelating extractant neutralizes the charge of the metal ion while the second extractant (synergistic agent) replaces the residual water from the inner coordination sphere of the central atom rendering the complex more hydrophobic. It was found also that at increased concentration of the synergistic agent the interaction between the two molecules is possible and then the reaction goes into the destruction of synergism region i.e. antagonistic effect occurs. This effect has been explained by a decrease of the available chelating agents concentration. Hence both synergistic and antagonistic effects depend on the experimental conditions of the extraction processes. The experiments for the synergistic extraction of Pr(III) with HTTA or HPMBP and Aliquat 336 in chloride and perchlorate form performed by our group to determine whether formation of ion-pairs Q+TTA− or Q+PMBP− obtained by the interaction, showed that at the experimental conditions of the extraction process such reaction is not significant. The important role of the quaternary salt anion (Cl− or ClO4−) on the extraction of lanthanoids (the change of Cl− with ClO4− causes a decrease of the overall equilibrium constant values with 3–4 orders of magnitude) was an additional proof that the above interaction is negligible. This trend is similar to that described for acidic–neutral ligand couples. Such an interaction if exists depends on the nature of the extractants, their concentration, diluent and composition of the aqueous phase i.e. not only on the experimental conditions, optimal or no. It have to be noted also that sometimes the experimental conditions at which the interaction is studied and the conditions at which the real metal extraction process is carried out differ and it is necessary to be taken into account. Nevertheless, contrarily to the common sense it seems like the opinion that the interaction between the extractants causes always antisynergistic effects is more and more plausible.Still, some major issues should be taken into account when choosing the better organic media as in dissolution processes all plausible multiple interactions between solutes and, solutes and “inert diluent” on the other hand may occur. Even thus, the search for more environmentally-benign diluents for extraction purposes is still in its infancy as an alternative choice of extremely high hydrophobic ILs is the only viable approach for now.30,134 It is mandatory to have in consideration all specific factors beforehand for each individual process addressing the economic and sustainable footprint of the whole performance.
Acknowledgements
Part of this work could be performed under the financial support of the FP7-PEOPLE Marie Curie Actions-IEF, project INNOVILLN (622906) as well as the Grant 11602/2016-UCTM.References
- Solvent extraction principles and practice, revised and expanded, ed. J. Rydberg, M. Cox, C. Musikas and G. Choppin, Marcel Deker, New York, 2004 Search PubMed.
- S. Khopkar, Solvent extraction-separation of elements with liquid ion exchangers, NewAge Sci., 2009 Search PubMed.
- F. Xie, T. Zhamg, D. Dreisinger and F. Doyle, Miner. Eng., 2014, 56, 10 CrossRef CAS.
- B. A. Moyer, Ion exchange and solvent extraction: a series of advances, CRC Press, Taylor&Francis group, 2010, vol. 19 Search PubMed.
- K. Nash and G. Choppin, Sep. Sci. Technol., 1997, 32, 255 CrossRef CAS.
- R. Sarkar, S. Ray and S. Basu, J. Chem., Biol. Phys. Sci., 2014, 4, 3156 CAS.
- A. Wilson, P. Bailey, P. Taskr, J. Turkington, R. Grant and J. Love, Chem. Soc. Rev., 2014, 43, 123 RSC.
- J. N. Mathur, Solvent Extr. Ion Exch., 1983, 1, 34 CrossRef.
- T. V. Healy, Synergistic adducts as coordination compounds, Proc. Int. Solv. Extr. Conf., Jerusalem, Israel, 1969, p. 257 Search PubMed.
- G. R. Choppin and A. Morgenstern, Solvent Extr. Ion Exch., 2000, 18, 1029 CrossRef CAS.
- P. Tahkur, J. L. Conca and G. R. Choppin, J. Solution Chem., 2012, 41, 599 CrossRef.
- J.-C. Bünzli, J. Coord. Chem., 2014, 67, 3706 CrossRef.
- G. R. Choppin, Sep. Sci. Technol., 1981, 16, 1113 CrossRef CAS.
- G. Kamble and M. Anuse, Synergistic extraction of coinage metals, LAP Lambert Academic Publ., 2015 Search PubMed.
- I. Dukov and M. Atanassova, High molecular weight amines and quaternary ammonium salts as synergistic agents in the solvent extraction of metal ions with chelating extractants, in Handbook of Inorganic Chemistry Research, ed. D. A. Morrison, Nova Science Publishers, 2010, ch. 7 Search PubMed.
- M. Atanassova, Solvent Extr. Ion Exch., 2009, 27, 159 CrossRef CAS.
- C. A. Blake, C. F. Baes, K. B. Brown, C. F. Coleman, and J. C. White, Solvent extraction of uranium and other metals by acidic and neutral organophosphorus compounds, Proceedings of the Second International Conference on the Peaceful Uses of Atomic Energy, IAEA, Geneva, Vienna, 1958, vol. 28, 1959, p. 289 Search PubMed.
- D. F. Peppard, G. W. Mason and R. J. Sironen, J. Inorg. Nucl. Chem., 1959, 10, 117 CrossRef CAS.
- T. V. Healy, D. F. Peppard and G. W. Mason, J. Inorg. Nucl. Chem., 1962, 24, 1429 CrossRef CAS.
- J. Ferraro and D. Peppard, J. Phys. Chem., 1961, 65, 539 CrossRef CAS.
- Y. Marcus and A. S. Kertes, in Ion exchange and solvent extraction of metal complexes, Willey Interscience, N.Y., 1969, p. 815 Search PubMed.
- G. Duyckaerts and J. F. Desreux, Int. Solv. Extr. Conf., (ISEC'77), Sept. 9–16, 1977, ed. B. H. Lucas, G. M. Ritcey and H. M. Smith, CIM Special, Toronto, 1979, vol. 21, pp. 73–85 Search PubMed.
- H. Freiser, Solvent Extr. Ion Exch., 1988, 6, 1093 CrossRef CAS.
- K. L. Nash, Studies of the Thermodynamics of Extraction of f-Elements, Solvent Extraction for 21st Century. Proc. of ISEC'99, Barcelona, Spain, July 11–16 1999, ed. M. Cox, M. Hidalgo and M. Valente, Publ. Soc. Chem. Ind., London, 2001, vol. 1, pp. 555–559 Search PubMed.
- K. Binnemans, Rare Earth Beta-Diketonates, in Handbook on the Physics and Chemistry of Rare Earths, ed. K. A. Gschneider, J. C. G. Bünzli and V. K. Perchasky, Elsevier, 2005, vol. 33, ch. 225, p. 107 Search PubMed.
- M. Atanassova and V. Kurteva, RSC Adv., 2016, 6, 11303 RSC.
- L. Bakos, S. Szabó, L. András and N. Iyer, Synergistic and antagonistic effects in organic solvent extraction of various metals, Proc. Symp. Coord. Chem., ed. M. Beck, Tihany, Hungary, 1965, p. 241 Search PubMed.
- I. Dukov, M. Atanassova, S. Zhivkova and A. Lirkov, J. UCTM, 2006, 41, 75 CAS.
- L. Song, S. Pan, L. Zhu, M. Wang, F. Du and J. Chen, Inorg. Chem., 2011, 50, 2215 CrossRef CAS PubMed.
- Z. Kolaric, Solvent Extr. Ion Exch., 2013, 31, 24 CrossRef.
- K. Binnemans, Lanthanides and actinides in ionic liquids, in Comprehensive Inorganic Chemistry II, ed. J. Reedijk and K. Peoppelmeir, Elsevier, Oxford, 2013, vol. 12, p. 641 Search PubMed.
- S. Shahriiari, L. Tomé, J. Araújo, L. Rebelo, J. Coutinho, I. Marrucho and M. Freire, RSC Adv., 2013, 3, 1835 RSC.
- M. Deetlefs, M. Fanselow and K. Seddon, RSC Adv., 2016, 6, 4280 RSC.
- A. Sengupta, P. Mohapatra, M. Iqbal, W. Verboom, J. Huskens and J. Godbole, RSC Adv., 2012, 2, 7492 RSC.
- S. Keavenery, J. Harper and A. Croft, RSC Adv., 2015, 5, 35709 RSC.
- V. Kurteva, M. Atanassova and I. Billard, J. Solution Chem., 2015, 44, 2416 CrossRef CAS.
- R. Ferreira, H. Garcia, A. Sousa, M. Petkovic, P. Lamosa, C. Freire, A. Silvestre, L. Rebelo and C. Pereira, New J. Chem., 2012, 36, 2014 RSC.
- R. Zirbs, K. Strassl, P. Gaertner, C. Schroder and K. Bica, RSC Adv., 2013, 3, 26010 RSC.
- B. Ribeiro, M. Coelho, L. Rebelo and I. Marrucho, Ind. Eng. Chem. Res., 2013, 52, 12146 CrossRef CAS.
- H. Passos, M. G. Freire and J. A. P. Coutinho, Green Chem., 2014, 16, 4786 RSC.
- V. Strehmel, S. Berdzinski and H. Rexhausen, J. Mol. Liq., 2014, 192, 153 CrossRef CAS.
- S. Khatun and E. W. Castner, J. Phys. Chem. B, 2015, 119, 9225 CrossRef CAS PubMed.
- C. Cannes, C. Naour, P. Moisy and P. Guilbaud, Inorg. Chem., 2013, 52, 11218 CrossRef CAS PubMed.
- Y. Li, J. Hu, M. Fu, J. Tang, L. Dong and S. Liu, RSC Adv., 2016, 6, 56772 RSC.
- L. Newman and P. Klotz, J. Phys. Chem., 1961, 65, 796 CrossRef CAS.
- L. Newman and P. Klotz, J. Phys. Chem., 1962, 66, 2262 CrossRef CAS.
- L. Newman and P. Klotz, Inorg. Chem., 1966, 5, 461 CrossRef CAS.
- L. Newman and P. Klotz, Inorg. Chem., 1972, 11, 2150 CrossRef CAS.
- L. Newman and P. Klotz, J. Phys. Chem., 1963, 67, 205 CrossRef.
- L. Newman, J. Inorg. Nucl. Chem., 1963, 25, 304 CrossRef CAS.
- C. H. Ke and N. C. Li, J. Inorg. Nucl. Chem., 1969, 31, 1383 CrossRef CAS.
- C. H. Ke and N. C. Li, Synergic Extraction of Cobalt(II) and Zink(II) with Thenoyltrifluoroacetone and Tri-n-Octylamine, in Solvent Extraction Research. Proc. Fifth Int. Conf. on Solv. Extr. Chem., Jerusalem, Israel, 16–18 Sept. 1968, ed. A. S. Kertes and Y. Marcus, Wiley-Interscience, New York, 1969, p. 281 Search PubMed.
- L. Genov and I. Dukov, Monatsh. Chem., 1973, 104, 750 CrossRef CAS.
- I. Dukov, A thesis submitted for the degree of DSc entitled “Investigations of the synergism in the solvent extraction of lanthanoids” (in Bulgarian), UCTM, Sofia, 1994, p. 251 Search PubMed.
- A. M. Poskanzer and B. M. Foreman, J. Inorg. Nucl. Chem., 1961, 16, 323 CrossRef CAS.
- Y. Lui and M. Lee, Solvent Extr. Ion Exch., 2016, 34, 74 CrossRef.
- Y. Liu and M. Lee, J. Mol. Liq., 2016, 220, 41 CrossRef CAS.
- H. F. Aly, M. Raieh, S. Mohamed and A. A. Abdel-Rassoul, J. Radioanal. Chem., 1977, 41, 65 CrossRef CAS.
- H. F. Aly, M. M. Dessouky, M. Raieh and S. Mohamed, J. Radioanal. Chem., 1978, 43, 7 CrossRef CAS.
- H. F. Aly, M. Raieh, S. Mohamed and A. A. Abdel-Rassoul, J. Inorg. Nucl. Chem., 1978, 40, 567 CrossRef CAS.
- S. M. Khalifa and H. F. Aly, J. Inorg. Nucl. Chem., 1980, 42, 1189 CrossRef CAS.
- M. N. Cheema, M. Mufazzal Saeed and I. H. Qureshi, Radiochim. Acta, 1980, 27, 109 CrossRef CAS.
- M. Mufazzal Saeed, M. N. Cheema and I. H. Qureshi, Radiochim. Acta, 1985, 38, 107 Search PubMed.
- M. Mufazzal Saeed, A. Ali, M. Ahmad, M. N. Cheema and I. H. Qureshi, J. Radioanal. Nucl. Chem., 1980, 129, 69 CrossRef.
- M. Mufazzal Saeed, A. Ali, M. Ahmad, M. N. Cheema and I. H. Qureshi, J. Radioanal. Nucl. Chem., 1989, 131, 149 CrossRef.
- M. Mufazzal Saeed, M. Ahmad, A. Ali and M. N. Cheema, Radiochim. Acta, 1992, 57, 125 Search PubMed.
- M. Mufazzal Saeed, M. Ahmad, A. Ali and M. N. Cheema, J. Radioanal. Nucl. Chem., 1992, 164, 1 CrossRef.
- L. Ch. Genov, I. L. Dukov and G. I. Kassabov, Acta Chim. Acad. Sci. Hung., 1977, 95, 361 CAS.
- I. L. Dukov and L. Genov, Acta Chim. Acad. Sci. Hung., 1980, 104, 329 CAS.
- M. Atanassova, V. M. Jordanov and I. L. Dukov, Hydrometallurgy, 2002, 63, 41 CrossRef CAS.
- I. L. Dukov and M. Atanassova, Hydrometallurgy, 2003, 68, 89 CrossRef CAS.
- M. Atanassova, V. M. Jordanov and I. L. Dukov, J. Univ. Chem. Technol. Metall., 2001, 36, 9 Search PubMed.
- P. K. Khopkar and J. N. Mathur, J. Inorg. Nucl. Chem., 1977, 39, 2063 CrossRef CAS.
- T. Sekine, R. Murai and H. Konno, Solvent Extr. Ion Exch., 1984, 2, 213 CrossRef CAS.
- T. Sekine, K. Tagaki and N. Dung, Bull. Chem. Soc. Jpn., 1993, 66, 2558 CrossRef CAS.
- T. Sekine, N. Dung and J. Noro, Bull. Chem. Soc. Jpn., 1994, 67, 432 CrossRef CAS.
- J. Noro and T. Sekine, Bull. Chem. Soc. Jpn., 1992, 65, 1910 CrossRef CAS.
- T. Sekine, H. Ishizaki, M. Hueda, R. Murai and S. Iwahori, Solvent Extr. Ion Exch., 1985, 3, 103 Search PubMed.
- J. Noro and T. Sekine, Bull. Chem. Soc. Jpn., 1992, 65, 2729 CrossRef CAS.
- J. Noro and T. Sekine, Bull. Chem. Soc. Jpn., 1993, 66, 804 CrossRef CAS.
- T. Sekine and N. Dung, Anal. Sci., 1993, 9, 851 CrossRef CAS.
- Y. Inoue, O. Tochiyama and I. Oda, J. Inorg. Nucl. Chem., 1979, 41, 1375 CrossRef CAS.
- H. Freiser, Solvent Extr. Ion Exch., 1988, 6, 1093 CrossRef CAS.
- O. Tochiyama and H. Freiser, Anal. Chim. Acta, 1981, 131, 233 CrossRef CAS.
- Y. Sasaki and H. Freiser, Inorg. Chem., 1983, 22, 2289 CrossRef CAS.
- J. P. Brunette, Z. Lakkis, M. Lakkis and M. J. F. Leroy, Proc. Int. Solv. Extr. Conf. (ISEC 83), Denver, Colorado, USA, 1983, p. 284 Search PubMed.
- J. P. Brunette, Z. Lakkis, M. Lakkis and M. J. F. Leroy, Polyhedron, 1985, 4, 577 CrossRef CAS.
- J. P. Brunette, M. Plea and M. J. F. Leroy, Solvent Extr. Ion Exch., 1984, 2, 1009 CAS.
- J. P. Brunette, M. Taheri, G. Goetz-Grandmont and M. J. F. Leroy, Polyhedron, 1982, 1, 457 CrossRef CAS.
- J. Kalembkiewicz, J. P. Brunette and M. J. F. Leroy, Monatsh. Chem., 1989, 120, 691 CrossRef CAS.
- M. Lakkis, Z. Lakkis, G. Goetz-Grandmont and J. P. Brunette, Monatsh. Chem., 1991, 122, 9 CrossRef CAS.
- Sight, G. Goetz-Grandmont and J. P. Brunette, Monatsh. Chem., 1992, 123, 373 CrossRef.
- K. Torkestani, G. Goetz-Grandmont and J. P. Brunette, Solvent Extr. Ion Exch., 1997, 15, 819 CrossRef CAS.
- S. Umetani, M. Matsui, H. Kawano and T. Nagai, Anal. Sci., 1985, 1, 55 CrossRef CAS.
- M. Mufazzal Saeed, M. A. Quyyum and A. Ali, Radiochim. Acta, 1997, 76, 205 Search PubMed.
- V. Jordanov, M. Atanassova and I. Dukov, J. UCTM, 2001, 2, 5 Search PubMed.
- I. Dukov, V. Jordanov and M. Atanassova, J. UCTM, 2003, 3, 853 Search PubMed.
- V. Schmit, Ekstraktziya aminami, Atomizdat, Moska, 1980 in Russian Search PubMed.
- I. L. Dukov, V. M. Jordanov and M. Atanassova, Solvent Extr. Ion Exch., 2001, 19, 619 CrossRef CAS.
- I. Dukov and L. Genov, J. Inorg. Nucl. Chem., 1981, 43, 412 CrossRef CAS.
- J. P. Brunette, M. Lakkis, G. Goetrz-Grandmont and M. J. F. Leroy, Polyhedron, 1982, 1, 461 CrossRef CAS.
- J. Kalembkiewicz, J. P. Brunette and M. J. F. Leroy, Solvent Extr. Ion Exch., 1988, 6, 919 CrossRef CAS.
- S. J. Kalenbkiewicz, M. J. F. Leroy and J. P. Brunette, Solvent Extr. Ion Exch., 1991, 9, 769 CrossRef.
- J. P. Brunette, Synergic Effects in Various Extraction Systems, in 4.Extraktionsseminar des Zentralinstitute für Festkörperphysik und Werkstofforschung, Holzlau, RDA, 11–14 Dec. 1989, Wissenschaftliche Berichte, 1990, vol. 44, p. 104 Search PubMed.
- A. Zhang, G. Wanyan and M. Kumagai, J. Solution Chem., 2004, 33, 1017 CrossRef CAS.
- A. Zhang, Solvent Extr. Ion Exch., 2001, 19, 925 CrossRef CAS.
- C. Deptula and S. Mine, J. Inorg. Nucl. Chem., 1967, 29, 159 CrossRef CAS.
- I. Fleitlikh, G. Pashkov, E. Stoyanov, I. Makarov, A. Kholkin, L. Nikiforova, N. Grigorieva, N. Pavlenko and G. Kolesnichenko, Solvent Extr. Ion Exch., 2002, 20, 765 CrossRef.
- M. Petrova, V. Kurteva and L. Lubenov, Ind. Eng. Chem. Res., 2011, 50, 12170 CrossRef CAS.
- D. Favaro and L. Atalla, J. Radioanal. Nucl. Chem., 1987, 111, 81 CrossRef CAS.
- W. D. Olieslager and L. Sannen, Solvent Extraction, ISEC'90, Kyoto, Japan 1990, July 16–21, ed. T. Sekine, Elsevier Science Publ. B.V., 1992, pp. 967–97 Search PubMed.
- C. F. Baes, Nucl. Sci. Eng., 1963, 16, 405 CAS.
- G. Goetz-Grandmont, M. Roqai, J. P. Brunette and G. Kaufmann, Solvent Extr. Ion Exch., 1996, 14, 653 CrossRef CAS.
- K. Kondo, T. Watanabe, M. Matsumoto and E. Kamio, J. Chem. Eng. Jpn., 2009, 42, 563 CrossRef CAS.
- M. L. P. Reddy, A. D. Damodaran, J. N. Mthur, M. S. Murali and R. H. Iyer, J. Radioanal. Nucl. Chem., 1995, 198, 367 CrossRef CAS.
- M. A. Petrova, ACS Sustainable Chem. Eng., 2016, 4, 2366 CrossRef CAS.
- J. Muller, C. Berthon, L. Couston, N. Zorz and J.-P. Simonin, Solvent Extr. Ion Exch., 2016, 34, 141 CrossRef CAS.
- V. K. Manchanda, P. N. Pathak and P. K. Mohapatra, Ion Exchange and Solvent Extraction A Series of Advances, ed. B. Moyer, CRC Press, Boca Raton, FL, 2010, vol. 19, p. 65 Search PubMed.
- D. Beltrami, G. Cote, H. Mokhtari, B. Courtaud and A. Chagnes, Hydrometallurgy, 2012, 129, 118 CrossRef.
- P. Tkac, G. Vandegrift, G. Lumetta and A. Gelis, Ind. Eng. Chem. Res., 2012, 51, 10433 CrossRef CAS.
- M. Atanassova, V. Kurteva, L. Lubenov, S. Varbanov and I. Dukov, Sep. Purif. Technol., 2012, 95, 58 CrossRef CAS.
- M. Atanassova, V. Kurteva, L. Lubenov, S. Varbanov and I. Billard, New J. Chem., 2015, 39, 7932 RSC.
- M. Atanassova, V. Kurteva, L. Lubenov and I. Billard, RSC Adv., 2014, 4, 38820 RSC.
- S. Banerjee, P. Dey and S. Basu, Radiochemistry, 2010, 52, 189 CrossRef CAS.
- F. A. Cotton and R. H. Holm, J. Am. Chem. Soc., 1960, 82, 2979 CrossRef CAS.
- I. L. Dukov, A. F. AI-Nimri and G. I. Kassabov, Monatsh. Chem., 1985, 116, 737 CrossRef CAS.
- S. A. Pai, K. V. Lohithakshan, P. D. Mithapara and S. K. Aggarwa, J. Radioanal. Nucl. Chem., 2000, 245, 623 CrossRef CAS.
- C. Hansch, in Physicochemical Aspects of Drug Action, ed. E. J. Ariens, Pergamon, London, 1968 Search PubMed.
- C. Hansch, Acc. Chem. Res., 1969, 2, 232 CrossRef CAS.
- C. Hansch, in Drug Design, ed. E. J. Ariens, Academic, New York, 1971 Search PubMed.
- J. H. Hildebrand and R. L. Scott, The Solubility of Nonelectrolytes, Dover, New York, 3rd edn, 1964 Search PubMed.
- F. A. Cotton and R. H. Holm, J. Am. Chem. Soc., 1960, 82, 2979 CrossRef CAS.
- D. McAlister, R. Chiarizia, M. Dietz, A. Herlinger and P. Zalupski, Solvent Extr. Ion Exch., 2002, 20, 447 CrossRef CAS.
- M. Atanassova, V. Mazan and I. Billard, ChemPhysChem, 2015, 16, 1703 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |