Preparation of Pickering emulsions with short, medium and long chain triacylglycerols stabilized by starch nanocrystals and their in vitro digestion properties
Rong Liang*a,
Yanwei Jianga,
Wally Yokoyamab,
Cheng Yanga,
Guangqun Caoa and
Fang Zhongc
aKey Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, P. R. China. E-mail: rongliang@jiangnan.edu.cn; Tel: +86-18651571425
bProcessed Food Research, Agricultural Research Service, USDA, Albany, CA 94710, USA
cKey Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Food Science and Technology, Jiangnan University, Wuxi 214122, P. R. China
First published on 13th October 2016
Abstract
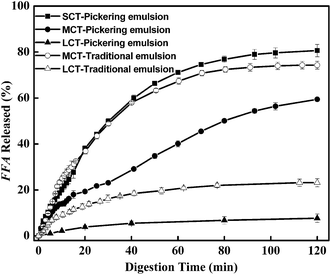
Food-grade Pickering emulsions are receiving more attention in the development of new delivery systems in food and pharmaceutical areas. In this study, starch nanocrystals (SNCs) prepared via acid hydrolysis of waxy maize starches were used as food-grade particle emulsifiers, to stabilize three typical edible oils with different chain lengths of fatty acid: short, medium and long chain triacylglycerols (SCT, MCT and LCT, respectively). On increasing the SNC concentrations from 0.1 to 3.0 wt%, the droplet size of these three Pickering emulsions decreased until 1.0 wt%. At this concentration, these three emulsions showed high stability against Ostwald ripening (for SCT) and coalescence (for MCT and LCT) for 90 days at 25 °C. However, the in vitro digestion properties of these three emulsions were significantly different. The rate of lipid digestion increased in the order LCT < MCT < SCT, whereas the percentage of SNC digested decreased in the order LCT > MCT > SCT. The inverse relationship between lipid and SNC hydrolysis rates may be due to, with the higher rate of lipid hydrolysis, more SNCs being displaced from the interface which would decrease the surface areas to enzymes. At the same time, calcium in digesta may absorb to SNCs to reduce the digestibility. These results may provide information about the physical and digestive properties of food-grade Pickering emulsions of different chain length triacylglycerols.
1. Introduction
The concept of emulsifiers was extended from amphiphilic molecules to particles by Ramsden in 1903 (ref. 1) and Pickering in 1907.2 They discovered that solid particles with suitable wetting contact angle between dispersed and continuous phases could stabilize emulsions. Particle stabilized emulsions became known as “Pickering emulsions”. Compared to traditional emulsions stabilized by molecular surfactants, Pickering emulsions exhibit outstanding long-term stability against coalescence and Ostwald ripening.3–5Various inorganic particles, such as silica,6 clay,7 and latex8 have been used as particle emulsifiers in Pickering emulsions. Some food components, such as fat crystals, casein micelles have been reported to be able to stabilize Pickering emulsions in food systems.9,10 Recently, common molecular emulsifiers (such as polysorbate 80) have been linked to metabolic syndrome and colitis by disrupting the mouse gut microbiota11 leading to safety concerns and the development of safe and natural emulsifiers for food and pharmaceutical applications. Particles of natural origin or food ingredients, such as amorphous cellulose,12 soy glycinin,13 whey protein nanoparticles,14 chitosan–tripolyphosphate nanoparticles,15 starch-based nanoparticles,16 micellar casein coated nanoemulsions17 and egg yolk granules18 have been fabricated and used as Pickering emulsifiers.
Starch-based particles have received increasing interest as Pickering emulsifiers because starch is safe, inexpensive, abundant and renewable natural polymers.19,20 In the past five years, several reports about the preparation and characteristics of Pickering emulsions stabilized by different types of starch-based particles have appeared.5,21–23 Timgren et al.24 compared the emulsion stabilizing capacity of different intact starch granules and found that size had more effect than shape on emulsion stability. Quinoa starch with the smallest size, 0.7–2.2 μm, was the best emulsifier. Our previous study agreed with these results showing that the stability of Pickering emulsions against coalescence was inversely proportional to the size of starch granules.25 Yusoff et al.5 explained that the increased stability of Pickering emulsions against coalescence or Ostwald ripening by smaller particles was due to their higher packing efficiency to produce a more homogenous layer around droplets.
In order to generate smaller size particles from starch that might improve the stability of Pickering emulsions, the amorphous regions of starch granules were hydrolyzed by acid leaving crystalline platelets. These crystalline products or starch nanocrystals (SNCs), have diameters of 47–118 nm with a thickness of 5–8 nm depending on the starch source.20 Previously we reported that SNCs from waxy maize, 40–100 nm, were able to emulsify liquid paraffin.26,27 The paraffin Pickering emulsions stabilized by waxy maize NC had particle size of ∼20 μm and were stable against coalescence for one year. In this study, we will investigate the stability of SNCs stabilized Pickering emulsions incorporating food-grade oils further.
According to the chain length of fatty acids, oils are normally categorized into short chain triacylglycerols (SCT) with C < 6,28 medium chain triacylglycerols (MCT) with C ∼ 6–12 (ref. 29) and long chain triacylglycerols (LCT) with C > 12.30 SCT, such as tributyrin, is essential for the healthy maintenance of intestinal functions by producing butyric acid in the body.31 MCT, predominately provided as caprylic/capric triglycerides in commercial, has been widely used for the dietary treatment of malabsorption syndrome, impaired lymphatic chylomicron transport and obesity, because of its rapid metabolism, fast satiety and low adipose tissue deposition.29,30,32 As for LCT, almost all vegetable oils are belong to LCTs, such as corn oil, olive oil and canola oil. They are the main sources for different fatty acids to our body.
Except for the inherent nutrition values, triacylglycerols of varying fatty acid chain length are commonly used as solvents or carriers for poorly soluble drugs or nutraceuticals in lipid-based delivery system.33,34 Ahmed et al.35 prepared curcumin emulsions by using SCT, MCT and LCT and revealed that the lipid digestion rate of SCT emulsions was highest, while the bioaccessibility of curcumin in SCT emulsions was lower than the ones with MCT and LCT by using in vitro models. Similar results were obtained by Salvia-Trujillo et al.36 and Qian et al.37 The reasons for the different rate of lipolysis and bioaccessibility of nutraceuticals may be attributed to the different digestive fate of lipids. With a relatively high water solubility, SCT is more accessible to contact and hydrolysis by pancreatic lipase. At the same time, low mobility of free fatty acids (FFAs) released from MCT and LCT would accumulated around oil droplets which further decreased the lipolysis.35 However, the mixed micelles formed by FFAs in MCT and LCT emulsions have a higher solubilization capacity for nutraceuticals than SCT emulsions. So with a compromised result, SCT emulsions with the higher lipolysis exhibited a lower bioaccessibility of nutraceuticals.38 The digestive fate of Pickering emulsions with different oils is still unknown until now. And compared to surfactant stabilized emulsions, Pickering emulsions may be effective to inhibit the instability caused by Ostwald ripening.39,40 So in this paper, Pickering emulsions incorporating SCT, MCT and LCT were first prepared and then the digestion properties were investigated.
Recently researches relating to the digestion fate of Pickering emulsions are mostly focused on the protein-based system.41–43 The digestion of starch-based Pickering emulsions are likely to be different and requires investigation. The results of this study will provide useful information about the physical and in vitro digestibility properties of Pickering emulsions stabilized by edible starch nanoparticles, as well as their potential to deliver functional oil or oil soluble nutraceuticals for foods and pharmaceuticals.
2. Materials and methods
2.1. Materials
Waxy maize starch and N-octenyl succinate anhydride (OSA) modified starch (CAPSUL) were kindly donated by Ingredion Co., Ltd. (Shanghai, China). Triacylglycerol oils with predominantly short, medium and long chain fatty acids (SCT, MCT and LCT, respectively) were used. Tributyrin (98%) (SCT) was purchased from Sigma-Aldrich Trading Co., Ltd. (Shanghai, China). Caprylic/capric triglycerides (MCT) with 43.7% C10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and 55.8% C8
0 and 55.8% C8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 was donated by Yihai Kerry Co., Ltd. (Shanghai, China). Canola oil (LCT) mainly with 63.0% C18
0 was donated by Yihai Kerry Co., Ltd. (Shanghai, China). Canola oil (LCT) mainly with 63.0% C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20.1% C18
1, 20.1% C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 9.6% C18
2, 9.6% C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, n-3, 6, 9, 3.5% C16
3, n-3, 6, 9, 3.5% C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1.5% C18
0, 1.5% C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and 1.4% C20
0 and 1.4% C20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was purchased from Standard foods Co., Ltd. (Shanghai, China) in a local supermarket. All oils were used without further purification. Enzymes used to simulate digestion: α-amylase (Aspergillus oryzae, P10065, 30 U mg−1), pancreatin (porcine pancreas, P7545, 8×USP specifications), bile salt extract (porcine, B8631), amyloglucosidase (Aspergillus niger, A7420, 316 U mL−1) and dyes (Nile red and Fluorescent brightener) were also purchased from Sigma-Aldrich Trading Co., Ltd (Shanghai, China) and used without further purification. All other chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). The D-glucose assay kit was purchased from Jiancheng Bioengineering Institute (Nanjing, China). Deionized water obtained from Milli-Q water purification system (Millipore Co., Bedford, MA, USA) was used in all experiments.
1 was purchased from Standard foods Co., Ltd. (Shanghai, China) in a local supermarket. All oils were used without further purification. Enzymes used to simulate digestion: α-amylase (Aspergillus oryzae, P10065, 30 U mg−1), pancreatin (porcine pancreas, P7545, 8×USP specifications), bile salt extract (porcine, B8631), amyloglucosidase (Aspergillus niger, A7420, 316 U mL−1) and dyes (Nile red and Fluorescent brightener) were also purchased from Sigma-Aldrich Trading Co., Ltd (Shanghai, China) and used without further purification. All other chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). The D-glucose assay kit was purchased from Jiancheng Bioengineering Institute (Nanjing, China). Deionized water obtained from Milli-Q water purification system (Millipore Co., Bedford, MA, USA) was used in all experiments.
2.2. Preparation of starch nanocrystals (SNCs)
SNCs were prepared from waxy maize starch (30 g) by hydrolysis with 3.16 M H2SO4 (150 mL) for 5 days at 40 °C with continuous stirring at 400 rpm as described by Angellier et al. with minor modification.44 After hydrolysis, the suspensions were washed with deionized water successively until the pH of supernatant separated by centrifugation was constant at 6.8. The resultant precipitate was dispersed in deionized water containing 0.02 wt% of NaN3 and stored at 4 °C. The SNC concentration of this stock solution was determined by weighing freeze-dried powders of the homogeneous dispersion by subtracting the weight of NaN3 added. Different concentrations of SNC suspensions were prepared by dilution from this stock homogeneous SNC dispersion using deionized water containing 0.02 wt% of NaN3.2.3. Characterizations of SNCs
The size and morphology of SNCs were observed by an environmental scanning electron microscope (ESEM) (S-4800, Hitachi, Japan) at high voltage (2.0 kV). SNC solution was prepared by ultrasonic and dropped on a copper mesh and then dried in ambient environment before observation.The structures of waxy maize starch and SNC were determined using a X-ray powder diffraction (XRD) (D8 ADVANCE, Bruker, Germany) with Cu Kα radiation (λ = 1.542 Å) at 40 kV and 40 mA. The X-ray diffraction patterns were recorded at a speed of 1° min−1 over the 2θ range of 3–35°. The relative crystallinity (RC) of samples was calculated according to the method from Kim et al.45 which was equal to the ratio of the area of crystalline to the sum of crystalline and amorphous.
2.4. Preparation of Pickering emulsions
Pickering emulsions stabilized by SNCs were prepared as described previously with minor modification.27 SNC dispersions with concentration from 0.1%, 0.5%, 1.0%, 1.5–3.0% (wt%) were prepared by serial dilution and stirred for at least 24 h. 5 mL of SNC dispersion was mixed with 5 mL of oil and the mixture was homogenized at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2.5 min at 25 °C using a high speed homogenizer (Ultra-Turrax T18, IKA Works Inc., Wilmington, NC).
000 rpm for 2.5 min at 25 °C using a high speed homogenizer (Ultra-Turrax T18, IKA Works Inc., Wilmington, NC).
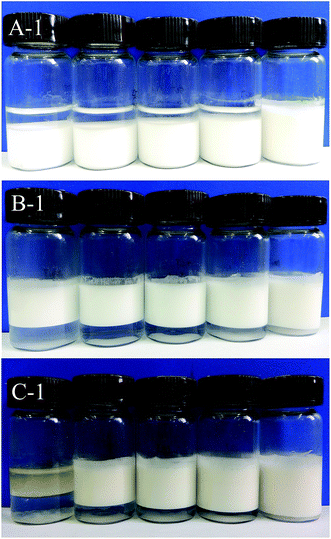
2.5. Stability of Pickering emulsions by visual inspection
The stability of emulsions to creaming and coalescence during storage was assessed by monitoring the position of the water-emulsion and emulsion–oil interfaces, respectively, as described by Binks et al.46 The appearance of Pickering emulsions in glass vessels were observed and photographed (Canon SD 4500 Digital camera) at various times at 25 °C.2.6. Optical microscopy of emulsions and droplet size measurements
According to Kalashnikova et al.,47 the droplet size of Pickering emulsions determined by light scattering and optical microscope are similar. So in this study, droplet size was determined by analysis of optical micrographs of the emulsions captured by a VHX-1000 digital microscope (Keyence Int. Trading Co. Ltd., Japan). The emulsion samples were diluted with water and then placed directly on a microscope slide. The droplet diameter was obtained by measuring over 200 droplets from the digital microscope images by using the VHX-1000C software installed in the instrument. According to the method from Timgren et al.24 and Zeeb et al.,48 the volume mean droplet diameter (D43), surface mean droplet diameter (D32) and number average droplet diameter (d10) were calculated using following functions (1)–(3).
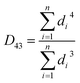 | (1) |
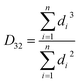 | (2) |
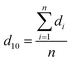 | (3) |
2.7. In vitro digestion
An in vitro model simulating digestion in the mouth, gastric and small intestine according to a procedure with slight modifications described by Braga et al.49 and Yi et al.50 was used in this study:(I) Initial system: the Pickering emulsions were mixed by vortex to ensure homogeneity with oil concentrations of 50% (v/v).
(II) Simulated mouth digestion: mixed solution containing various inorganic salts (0.896 g L−1 of KCl, 0.2 g L−1 of KSCN, 0.888 g L−1 of NaH2PO4, 0.57 g L−1 of Na2SO4, 0.298 g L−1 of NaCl and 1.694 g L−1 of NaHCO3) and organic component (0.2 g L−1 of urea) was first prepared. Later, simulated salivary fluid (SSF) was finished by adding bioactive components of α-amylase (0.6 g L−1) and uric acid (0.015 g L−1) into the mixed solution according to a previous study.49 The initial emulsion samples (4 mL) were mixed with 4 mL of SSF. The pH of the mixture was adjusted to 6.8 and stirred at 100 rpm for 3 min in a water jacketed beaker at 37 °C. The oil concentration for this step was 25% (v/v).
(III) Simulated gastric digestion: 10 mL of simulated gastric fluid (SGF) was added to the mixture after the simulated salivary digestion. The SGF was prepared by placing 2 g of NaCl and 7 mL of HCl (37 wt%) in 1 L of water and adjusting pH to 1.2 using 1.0 M HCl.51 The SGF-emulsion mixture was immediately adjusted to pH 2.0, and stirred at 100 rpm for 1 h at 37 °C. The oil concentration for this step was 11% (v/v). Pepsin was not included in the SGF because the glucose in pepsin would interfere with the analysis of SNC digestion. And according to Woolnough et al.,52 pepsin does not influence the digestion in starch-based system.
(IV) Simulated intestinal digestion: after the gastric digestion step, the samples were immediately adjusted to pH 7.0 by using 1 M NaOH. 15 mL of simulated intestinal fluid (SIF) composed of 1 mL of CaCl2 (165 mM), 13.2 mL of freshly made pancreatin (6 mg mL−1) and bile salt (50 mg mL−1) solution, 20 μL of amyloglucosidase (AMG) and 780 μL of deionized water was added according to the method from Mahasukhonthachat et al.53 The final mixture contained 6.06% (v/v) oil, 2.4 mg mL−1 of pancreatin, 20.0 mg mL−1 bile extract, 5 mM CaCl2 and 0.18 U mL−1 AMG, respectively.
At the start of the intestinal digestion process, pH of the digesta immediately decreases due to production of fatty acids by the lipase hydrolysis of triglycerides. The rate of fat hydrolysis was determined by maintaining the pH at 7.0 by adding NaOH (0.5 M) manually using pipette and the amount of NaOH added over time (120 min) was recorded by computer. The percentage of FFA released during the digestion was calculated according to the volume of NaOH solution added at each time point by the following equation:
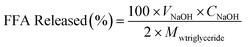 | (4) |
2.8. SNC digestion
SNCs were hydrolyzed during in vitro digestion by amylase and amyloglucosidase. The concentration of glucose released from SIF digestion of SNCs at time intervals was quantified using a D-glucose assay kit containing glucose oxidase and peroxidase (GOPOD) according to the method from Ai et al.54 The digesta samples were placed in a water bath at 90 °C for 5 min to inactive the enzyme. The absorbance of standards and samples against blank was measured at 505 nm. The percentage of SNC digested was calculated using equation:
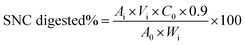 | (5) |
2.9. Fluorescence microscope
An upright fluorescence microscope (Nikon, Fluorescent DIC/500 pixels, Japan) with a 10× objective lens was used to capture images of the emulsions during the digestion process. Fluorescence of the lipid phase was generated by Nile red (0.05 wt% dissolved in ethanol), excited at 488 nm by argon laser light. Fluorescence bright (0.01 wt% dissolved in water), as a fluorescent dye for SNCs, was excited at 405 nm by argon laser light.2.10. Statistical analysis
The entire experiment was conducted in duplicate and all analyses were carried out at least in triplicate and data are expressed as mean ± standard deviation (SD). Data was analyzed by One Way Analysis of Variance (ANOVA) test using the statistical software package SPSS 17.0. Duncan's multiple range test was used to determine significant differences between mean values (p < 0.05).3. Results and discussion
3.1. Characterizations of SNCs
SNCs were prepared by means of sulfuric acid hydrolysis of waxy maize starches for 5 days and their morphology was observed by SEM. As shown in Fig. 1A, the structure of SNCs was polygonal and their size ranged from 40 to 100 nm. But SNCs were easily self-aggregated by forming agglomerates,55,56 which was confirmed with the aggregates with size larger than 100 nm observed in SEM image. This result of SEM observation was in agreement with publications from other researchers.55,57 According to our previous work,27 the size distribution of SNCs dispersion determined by dynamic light scattering was wide and the average size was about 120 nm. This results were consistent with the observation by SEM. | ||
| Fig. 1 (A) SEM image of SNCs with acid hydrolysis time of 5 days. (B) XRD patterns of native waxy maize starches and SNCs with acid hydrolysis time of 5 days. | ||
Powder X-ray diffraction was used to investigate the crystalline structure of SNCs. The XRD patterns of waxy maize starches and SNCs were given in Fig. 1B. Waxy maize starches and SNCs showed a similar diffraction pattern with strong peaks at 15° and 23° and unresolved double peaks at 17° and 18°, as well as two weak peaks around 10° and 11° of 2θ representing both of them had typical A-type crystalline arrangement. This results were in good agreement with the investigates from Kim et al.45,58 and Ren et al.,56 which indicated that acid hydrolysis of waxy maize starches did not destroy the crystalline pattern of starches. Compared to waxy maize starches, the RC value of SNCs was increased from 36.4% to 44.3% which was consistent with the previous works.19,58 The reason for this result was assumed to be cause by the loss of amorphous regions in starch granules and reordering of the residuals to a more ordered arrangement during the acid hydrolysis.19
3.2. Preparation of Pickering emulsions with different types of oil
In addition, the photographs showed that with increasing of SNC concentration, the height of the emulsion layer increased which means the stability of the emulsions against creaming increased. However, higher SNC concentrations would result in the emulsions to form gel-like structure and in order to avoid this situation, the concentration of SNCs was kept below 6.0 wt%.26
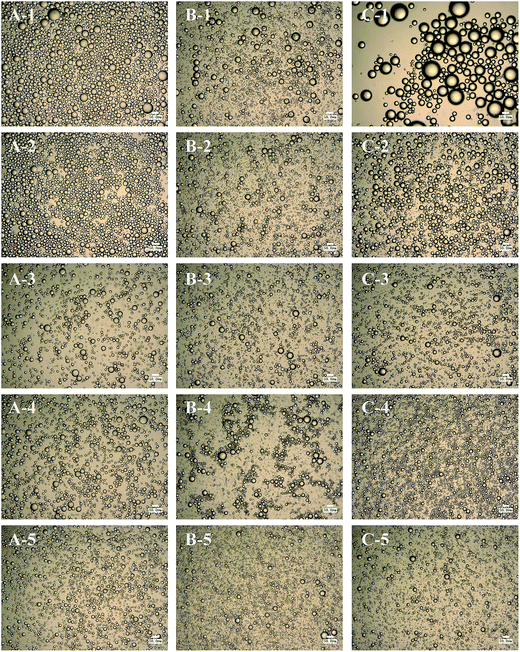
Non-absorbed SNC aggregates were clearly observed in the continuous phase at higher (1.0 wt%) SNC concentrations in Pickering emulsions incorporating SCT at 1000× magnification (Fig. 4A). Increasing SNC concentrations to 1.5 wt% and 3.0 wt%, the amount of non-absorbed SNC aggregates increased (Fig. 4B and C). The observation of particles in the continuous phase was also observed when microcrystalline cellulose was used as the particle stabilizer.23 However, free SNC aggregates were not observed in the continuous phase for all SNC concentrations of MCT and LCT emulsions. These results were in agreement with previous work on Pickering emulsions incorporating oils with different polarity stabilized by silica particles by Frelichowska et al.60 They found that more particles are required for less polar oils, just like MCT and LCT in our research. The differences of the interfacial properties of these three emulsions may further influence their in vitro digestion properties.
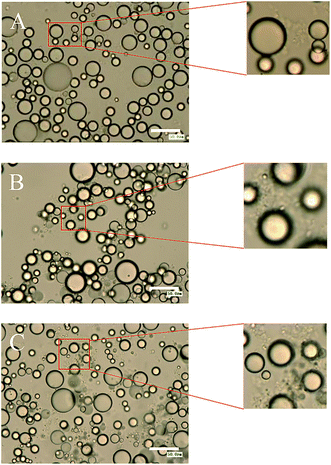 | ||
| Fig. 4 The effect of SNCs concentration of 1.0 wt% (A), 1.5 wt% (B) and 3.0 wt% (C) on the optical microscope images of emulsions incorporating SCT. The scale bar is 50 μm. | ||
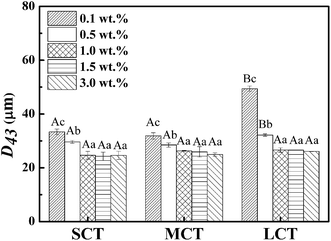
The volume mean droplet diameters (D43) of the emulsions stabilized at various concentrations of SNCs analyzed by light microscope were shown in Fig. 5. The larger D43 value of LCT at 0.1 wt% of SNCs corresponded to emulsion separation observed visually in Fig. 2C. Overall, the D43 values of all the SCT, MCT and LCT Pickering emulsions decreased with increasing SNC concentrations from 0.1 to 1.0 wt%, and then remained constant (p > 0.05). These results were in good agreement with the research of Pickering emulsions stabilized by starch-based nanoparticles reported by Tan et al.21 They also found that at 1.25 to 2.0 wt% of starch nanoparticles, the emulsion droplet size did not decrease further and was about 39 μm. The reason for this phenomenon was attributed to the limitation of homogenization conditions.22 Furthermore, with the increasing of SNC concentrations, the viscosity of emulsion systems increased, which decrease the efficiency of homogenization again.12 Since non-absorbed SNC aggregates were observed in SCT emulsions above 1.0 wt% SNCs and all emulsions were shown to have the similar minimum particle size above 1.0 wt%, 1.0 wt% SNC was determined to be the minimum amount necessary to completely emulsify the oils under this emulsification conditions. And 1.0 wt% SNCs concentration was used in subsequent experiments.
| Oil type | Day 1 | Day 7 | Day 30 | Day 90 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D43 (μm) | D32 (μm) | d10 (μm) | D43 (μm) | D32 (μm) | d10 (μm) | D43 (μm) | D32 (μm) | d10 (μm) | D43 (μm) | D32 (μm) | d10 (μm) | |
| a Data expressed as mean ± standard deviation (n = 3). | ||||||||||||
| SCT | 24.65 ± 0.07 | 21.63 ± 0.18 | 16.84 ± 0.06 | 30.97 ± 1.52 | 27.53 ± 1.52 | 17.15 ± 0.03 | 34.79 ± 0.12 | 29.62 ± 0.45 | 17.82 ± 0.83 | 35.31 ± 1.73 | 30.22 ± 1.67 | 18.69 ± 0.58 |
| MCT | 26.27 ± 0.18 | 22.82 ± 0.12 | 13.33 ± 0.46 | 26.02 ± 0.21 | 22.31 ± 0.55 | 13.30 ± 0.29 | 25.91 ± 0.31 | 22.31 ± 0.42 | 13.28 ± 1.08 | 26.13 ± 0.31 | 23.20 ± 1.45 | 13.33 ± 0.14 |
| LCT | 26.60 ± 0.81 | 23.11 ± 1.44 | 15.59 ± 1.45 | 26.77 ± 0.30 | 22.66 ± 1.64 | 15.90 ± 0.14 | 26.33 ± 0.84 | 23.34 ± 1.07 | 15.89 ± 0.08 | 26.57 ± 0.84 | 23.68 ± 0.24 | 15.91 ± 0.33 |
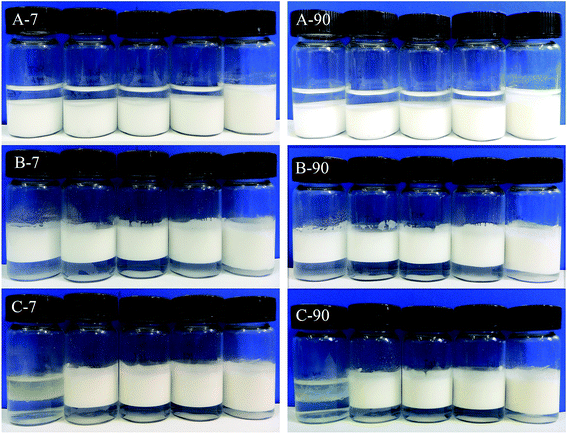
Photographs of the emulsions at Day 7 and Day 90 were shown in Fig. 7. Compared to the emulsions in Fig. 2, no significant changes were perceived except for the creaming phenomenon for MCT and LCT Pickering emulsions and sedimentation for SCT Pickering emulsions. The droplet size and visual assessment of the storage stability showed that at 1.0 wt% SNC, Pickering emulsions exhibit outstanding stability against coalescence (for MCT and LCT) and Ostwald ripening (for SCT) at 25 °C for 90 days.
3.3. In vitro digestion of SNC stabilized Pickering emulsions
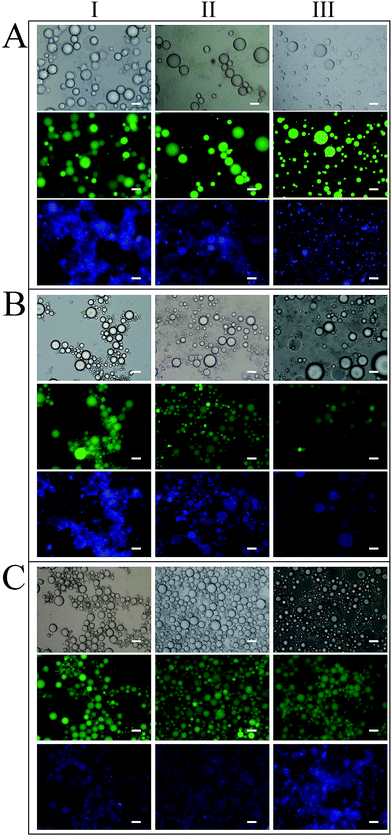
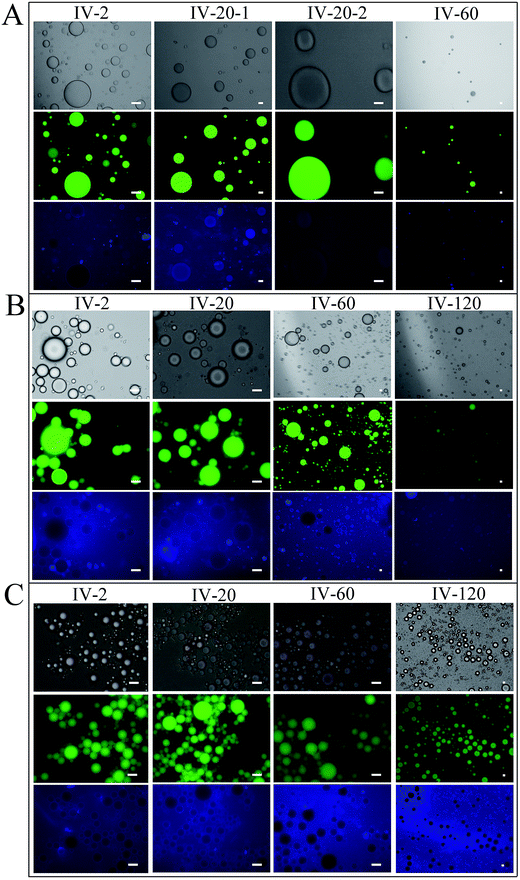
There were no significant changes in the morphology and droplet size of any Pickering emulsions after incubation in SSF for 3 min (Fig. 8A-II, B-II and C-II). After the gastric digestion process for 1 h, the size of particles incorporating SCT and MCT became less uniform and particles incorporating LCT appeared to flocculate as shown by the continuous blue fluorescence in the images (Fig. 8A-III, B-III and C-III). These phenomenon may have been caused by a decrease in the negative charge of the sulfate groups at the surface of SNCs at low pH in SGF. Li et al.27 reported that the zeta potential of SNCs decreased with increasing pH and at pH 3–4, liquid paraffin Pickering emulsions stabilized by SNCs aggregated. However, the biggest differences between Pickering emulsions with different lipids occurred during the simulated small intestinal stage. Photographs of samples in SIF after 2, 20, 60 and 120 min were shown in Fig. 9.
Large droplets were observed for Pickering emulsions with SCT after 2 and 20 min (Fig. 9A-IV-2 and IV-20) in SIF, but after 60 min large droplets disappeared and after 120 min little undigested oil remained. With little droplets left in photos of SCT emulsions in SIF after 120 min, so they were not exhibited in Fig. 9A. Droplet changes in MCT Pickering emulsions were similar to SCT, however, large droplets remained after 60 min and undigested oil was still present after 120 min in SIF. The oil droplets of LCT Pickering emulsions in SIF became larger when they were incubated in SIF. But with increasing digestion time in SIF, the oil droplet size increased negligibly.
The SNCs adsorbed to the oil droplets also changed during the SIF digestion. In the initial emulsions, SNCs appeared as a halo around the oil droplets (Fig. 8A-I, B-I and C-I). Incubation of the SCT emulsions in SIF for 20 min, resulted in disappearance of the blue halo of SNC around the oil droplets as shown by images with higher magnification (IV-20-2 in Fig. 9A). The fluorescence of the SNC blue halo also became fainter with increasing of digestion time for MCT and LCT. At 120 min in SIF the blue halo in the LCT emulsions disappeared completely (IV-120 in Fig. 9C).
In order to investigate the reason for the differences of the microstructure changes of these three Pickering emulsions, the hydrolysis profiles of lipid and SNC during in vitro digestion were analyzed.
| Emulsion type | Oil type | D32 (μm) | Specific surface area (m2) | Initial rate of lipolysis (min−1 × 102) |
|---|---|---|---|---|
| a Data expressed as mean ± standard deviation (n = 3). Values of means followed by different uppercase capital letters (A–E) in the same columns are significantly different (p < 0.05). | ||||
| Pickering emulsion | SCT | 21.63 ± 0.18B | 0.1387 ± 0.0012B | 1.94 ± 0.10D |
| MCT | 22.82 ± 0.12B | 0.1314 ± 0.0007B | 1.34 ± 0.09C | |
| LCT | 23.11 ± 1.44B | 0.1300 ± 0.0081B | 0.22 ± 0.05A | |
| Traditional emulsion | MCT | 0.20 ± 0.01A | 14.69 ± 0.56A | 2.63 ± 0.48E |
| LCT | 0.21 ± 0.01A | 14.58 ± 0.28A | 0.94 ± 0.09B | |
However, no significant differences (p > 0.05) for specific surface areas among three Pickering emulsions were found before digestion and similar results were also obtained for traditional emulsions. The different fate of lipid digestion profiles for these emulsions was most likely caused by the different properties of the triglycerides. Either for Pickering emulsions nor traditional emulsions, emulsions with LCT exhibited lower rate and extant of lipolysis in comparison to MCT emulsions. For SCT, although the initial rate of SCT lipolysis in Pickering emulsions was lower than MCT in traditional emulsions, the final extent of SCT lipolysis in Pickering emulsions was still higher than MCT traditional emulsions. Two factors are thought to be mainly responsible. Firstly, SCT with a relatively high water solubility was more accessible to contact and hydrolysis by pancreatic lipase in water system.38 Secondly, short chain and medium chain FFA digestion products produced from SCT and MCT diffuse more easily into the surrounding aqueous phase than the long chain FFA digestion products arising from LCT which further decrease the contact of lipase to oil droplets in LCT emulsions.35
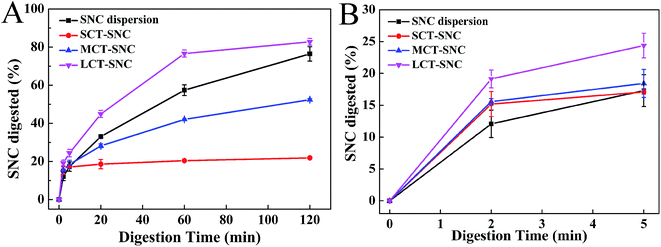
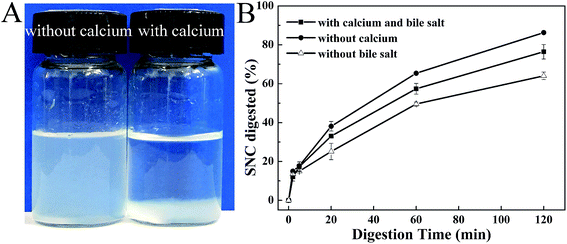
According to the previous work, the aggregation of SNCs was affected by salt.27 So in SIF calcium may also impact the dispersity of SNCs. As shown in Fig. 12A, compared to SNC water dispersion, with the addition of calcium, precipitates formed by calcium and SNCs were easily separated from the system. Furthermore, specific digestion processes without calcium and bile salt in SIF for SNC dispersions were carried out to clarify if the aggregates would affect the SNC digestion. As the results shown in Fig. 12B, without calcium in SIF the digestion of SNCs increased significantly. Conversely, without bile salt in SIF the digestion of SNCs decreased obviously. These results could be inferred that the aggregates formed by calcium and SNCs would decrease the SNC digestion. With no bile salt, calcium would not be used to precipitate bile salt and more calcium in the system would interact with SNCs and further to decrease the SNC digestion rate. However, for the digestion of Pickering emulsions, FFA released from MCT and LCT lipolysis would also consume calcium by forming soaps. So the interaction of calcium with either SNC, bile salt or FFA was though be the main reason for the different rates of SNC digestion.
The water dispersion of SNCs contains no FFA in system, calcium is available to precipitate bile salts and SNCs. Therefore, the rate of digestion of SNC was not reduced by its partial reaction with calcium and the extent of digestion increased with digestion time. In the case of SCT Pickering emulsions, although a greater amount of FFA was released by lipolysis (Fig. 10), short chain fatty acids are not readily precipitated by calcium63 and bile salts would replace the SNCs at the interface (as shown in Fig. 9A, IV-20-2, third row). Therefore, more calcium would be available to precipitate SNCs including the displaced and originally unbound SNCs aggregates in the continuous phase (Fig. 4A), which corresponded to the depressed SNC digestion after 20 min. Calcium forms soaps with the FFA produced from MCT digestion, so the amount of calcium available to form precipitates with SNCs decreased which would increase the extent of SNC digestion of MCT Pickering emulsions compared to SCT emulsions. For LCT Pickering emulsions, FFA would form soaps and decrease calcium precipitation of SNCs. With the low rate of lipid hydrolysis (Fig. 10) the droplet size of Pickering emulsions remained relatively constant as shown in Fig. 9C. So SNCs adsorbed at the oil–water interface would have a great surface area to enzymes to increase the SNC digestion rate to 85% after 120 min in SIF.
4. Conclusion
In summary, Pickering emulsions stabilized by SNCs at 1.0 wt% showed outstanding stability against Ostwald ripening by incorporating SCT and exhibited high stability against coalescence by incorporating MCT and LCT at 25 °C for 90 days. The in vitro digestion properties of these three emulsions were significantly different. The rate of FFA released was in the order SCT > MCT > LCT and the extent of SNC digested was in the order SCT < MCT < LCT. The inverse relationship between triglyceride and SNC hydrolysis rates may be related to the interaction of calcium with SNC, bile salt or FFA and the specific surface areas during digestion. With lipid hydrolysis, the SNCs were displaced from the interface and calcium in digesta would adsorb to SNCs to decrease their hydrolysis. But the calcium available to interact with SNCs changes as the properties and amount of the FFA produced from hydrolysis of SCT, MCT and LCT and bile salts in SIF. At the same time, with low lipolysis for LCT Pickering emulsions, more specific surface areas were available for SNCs to contact with enzymes which further to increase the SNC digestion significantly. So the characteristics of both lipid and emulsifier affect the digestive fate of Pickering emulsions. These results may be useful for developing Pickering emulsions with different digestion profiles by adjusting the components in the formulations for food and pharmaceuticals.Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 31401533, 31571891) and the National Thirteenth-Five Year Research Program of China (2016YFD0400801) and the Fundamental Research Funds for the Central Universities (No. JUSRP11422, JUSRP51507).References
- W. Ramsden, Proc. R. Soc. London, 1903, 72, 156–164 CrossRef CAS.
- S. U. Pickering, J. Chem. Soc., Trans., 1907, 91, 2001–2021 RSC.
- B. P. Binks, Curr. Opin. Colloid Interface Sci., 2002, 7, 21–41 CrossRef CAS.
- R. Aveyard, B. P. Binks and J. H. Clint, Adv. Colloid Interface Sci., 2003, 100–102, 503–546 CrossRef CAS.
- A. Yusoff and B. S. Murray, Food Hydrocolloids, 2011, 25, 42–55 CrossRef CAS.
- B. P. Binks and C. P. Whitby, Langmuir, 2004, 20, 1130–1137 CrossRef CAS PubMed.
- Y. Nonomura and N. Kobayashi, J. Colloid Interface Sci., 2009, 330, 463–466 CrossRef CAS PubMed.
- B. P. Binks and S. O. Lumsdon, Langmuir, 2001, 17, 4540–4547 CrossRef CAS.
- E. Dickinson, Curr. Opin. Colloid Interface Sci., 2010, 15, 40–49 CrossRef CAS.
- E. Dickinson, Trends Food Sci. Technol., 2012, 24, 4–12 CrossRef CAS.
- B. Chassaing, O. Koren, J. K. Goodrich, A. C. Poole, S. Srinivasan, R. E. Ley and A. T. Gewirtz, Nature, 2015, 519, 92–96 CrossRef CAS PubMed.
- X. Jia, R. Xu, W. Shen, M. Xie, M. Abid, S. Jabbar, P. Wang, X. Zeng and T. Wu, Food Hydrocolloids, 2015, 43, 275–282 CrossRef CAS.
- F. Liu and C.-H. Tang, Food Hydrocolloids, 2016, 60, 620–630 CrossRef CAS.
- J. Wu, M. Shi, W. Li, L. Zhao, Z. Wang, X. Yan, W. Norde and Y. Li, Colloids Surf., B, 2015, 127, 96–104 CrossRef CAS PubMed.
- B. R. Shah, Y. Li, W. Jin, Y. An, L. He, Z. Li, W. Xu and B. Li, Food Hydrocolloids, 2016, 52, 369–377 CrossRef CAS.
- K. Lu, M. Miao, F. Ye, S. W. Cui, X. Li and B. Jiang, Carbohydr. Polym., 2016, 147, 392–400 CrossRef CAS PubMed.
- A. Ye, X. Zhu and H. Singh, Langmuir, 2013, 29, 14403–14410 CrossRef CAS PubMed.
- M. Rayner, D. Marku, M. Eriksson, M. Sjöö, P. Dejmek and M. Wahlgren, Colloids Surf., A, 2014, 458, 48–62 CrossRef CAS.
- H.-Y. Kim, J.-A. Han, D.-K. Kweon, J.-D. Park and S.-T. Lim, Carbohydr. Polym., 2013, 93, 582–588 CrossRef CAS PubMed.
- D. LeCorre, J. Bras and A. Dufresne, Carbohydr. Polym., 2012, 87, 658–666 CrossRef CAS.
- Y. Tan, K. Xu, C. Niu, C. Liu, Y. Li, P. Wang and B. P. Binks, Food Hydrocolloids, 2014, 36, 70–75 CrossRef CAS.
- M. Rayner, A. Timgren, M. Sjöö and P. Dejmek, J. Sci. Food Agric., 2012, 92, 1841–1847 CrossRef CAS PubMed.
- M. Kargar, K. Fayazmanesh, M. Alavi, F. Spyropoulos and I. T. Norton, J. Colloid Interface Sci., 2012, 366, 209–215 CrossRef CAS PubMed.
- A. Timgren, M. Rayner, P. Dejmek, D. Marku and M. Sjöö, Food Sci. Nutr., 2013, 1, 157–171 CrossRef CAS PubMed.
- C. Li, Y. Li, P. Sun and C. Yang, Colloids Surf., A, 2013, 431, 142–149 CrossRef CAS.
- C. Li, P. Sun and C. Yang, Starch/Staerke, 2012, 64, 497–502 CrossRef CAS.
- C. Li, Y. Li, P. Sun and C. Yang, J. Sci. Food Agric., 2014, 94, 1802–1807 CrossRef CAS PubMed.
- O. C. Velazquez, R. W. Seto and J. L. Rombeau, Proc. Nutr. Soc., 1996, 55, 49–78 CrossRef CAS PubMed.
- B. Marten, M. Pfeuffer and J. Schrezenmeir, Int. Dairy J., 2006, 16, 1374–1382 CrossRef CAS.
- M.-P. St-Onge and P. J. H. Jones, J. Nutr., 2002, 132, 329–332 CAS.
- J. D. Donovan, S.-Y. Lee and Y. Lee, J. Food Sci., 2016, 81, S2252–S2257 CrossRef CAS PubMed.
- K. Nagao and T. Yanagita, Pharmacol. Res., 2010, 61, 208–212 CrossRef CAS PubMed.
- S. M. Caliph, W. N. Charman and C. J. H. Porter, J. Pharm. Sci., 2000, 89, 1073–1084 CrossRef CAS PubMed.
- Q. Xing, J. Song, X. You, D. Xu, K. Wang, J. Song, Q. Guo, P. Li, C. Wu and H. Hu, Int. J. Pharm., 2016, 511, 709–718 CrossRef CAS PubMed.
- K. Ahmed, Y. Li, D. J. McClements and H. Xiao, Food Chem., 2012, 132, 799–807 CrossRef CAS.
- L. Salvia-Trujillo, Q. Sun, B. H. Um, Y. Park and D. J. McClements, J. Funct. Foods, 2015, 17, 293–304 CrossRef CAS.
- C. Qian, E. A. Decker, H. Xiao and D. J. McClements, Food Chem., 2012, 135, 1440–1447 CrossRef CAS PubMed.
- Y. Li, M. Hu, Y. Du, H. Xiao and D. J. McClements, Food Hydrocolloids, 2011, 25, 122–130 CrossRef CAS.
- Y. Li, S. Le Maux, H. Xiao and D. J. McClements, J. Agric. Food Chem., 2009, 57, 9243–9249 CrossRef CAS PubMed.
- J. A. Juárez and C. P. Whitby, J. Colloid Interface Sci., 2012, 368, 319–325 CrossRef PubMed.
- E. Filippidi, A. R. Patel, E. C. M. Bouwens, P. Voudouris and K. P. Velikov, Adv. Funct. Mater., 2014, 24, 5962–5968 CrossRef CAS.
- F. Liu and C.-H. Tang, Food Hydrocolloids, 2016, 56, 434–444 CrossRef CAS.
- Y. Shao and C.-H. Tang, Food Res. Int., 2016, 79, 64–72 CrossRef CAS.
- H. Angellier, L. Choisnard, S. Molina-Boisseau, P. Ozil and A. Dufresne, Biomacromolecules, 2004, 5, 1545–1551 CrossRef CAS PubMed.
- H.-Y. Kim, D. J. Park, J.-Y. Kim and S.-T. Lim, Carbohydr. Polym., 2013, 98, 295–301 CrossRef CAS PubMed.
- B. P. Binks and J. A. Rodrigues, Langmuir, 2007, 23, 7436–7439 CrossRef CAS PubMed.
- I. Kalashnikova, H. Bizot, B. Cathala and I. Capron, Langmuir, 2011, 27, 7471–7479 CrossRef CAS PubMed.
- B. Zeeb, M. Gibis, L. Fischer and J. Weiss, J. Colloid Interface Sci., 2012, 387, 65–73 CrossRef CAS PubMed.
- A. C. Braga, R. N. Alves, A. L. Maulvault, V. Barbosa, A. Marques and P. R. Costa, Food Chem. Toxicol., 2016, 89, 54–59 CrossRef CAS PubMed.
- J. Yi, Y. Li, F. Zhong and W. Yokoyama, Food Hydrocolloids, 2014, 35, 19–27 CrossRef CAS.
- A. Sarkar, K. K. T. Goh, R. P. Singh and H. Singh, Food Hydrocolloids, 2009, 23, 1563–1569 CrossRef CAS.
- J. W. Woolnough, J. A. Monro, C. S. Brennan and A. R. Bird, Int. J. Food Sci. Technol., 2008, 43, 2245–2256 CrossRef CAS.
- K. Mahasukhonthachat, P. A. Sopade and M. J. Gidley, J. Food Eng., 2010, 96, 18–28 CrossRef.
- Y. Ai, B. Nelson, D. F. Birt and J.-l. Jane, Carbohydr. Polym., 2013, 98, 1266–1271 CrossRef CAS PubMed.
- B. Wei, X. Hu, H. Li, C. Wu, X. Xu, Z. Jin and Y. Tian, Food Hydrocolloids, 2014, 36, 369–373 CrossRef CAS.
- L. Ren, M. Jiang, L. Wang, J. Zhou and J. Tong, Carbohydr. Polym., 2012, 87, 1874–1876 CrossRef CAS.
- D. LeCorre, J. Bras and A. Dufresne, Biomacromolecules, 2011, 12, 3039–3046 CrossRef CAS PubMed.
- H.-Y. Kim, J. H. Lee, J.-Y. Kim, W.-J. Lim and S.-T. Lim, Starch/Staerke, 2012, 64, 367–373 CrossRef CAS.
- D. J. McClements and J. Rao, Crit. Rev. Food Sci. Nutr., 2011, 51, 285–330 CrossRef CAS PubMed.
- J. Frelichowska, M.-A. Bolzinger and Y. Chevalier, Colloids Surf., A, 2009, 343, 70–74 CrossRef CAS.
- E. Dickinson, C. Ritzoulis, Y. Yamamoto and H. Logan, Colloids Surf., B, 1999, 12, 139–146 CrossRef CAS.
- G. Saravacos, P. Taoukis, M. Krokida, V. Karathanos, H. Lazarides, N. Stoforos, C. Tzia, S. Yanniotis, A. Timgren, M. Rayner, M. Sjöö and P. Dejmek, Procedia Food Sci., 2011, 1, 95–103 CrossRef.
- D. G. Fatouros and A. Mullertz, Expert Opin. Drug Metab. Toxicol., 2007, 4, 65–76 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |