A benzoperylene probe self-assembled on polyethyleneimine/manganese doped ZnS quantum dots: a new nanocomposite for bright and tunable white light emission†
Abstract
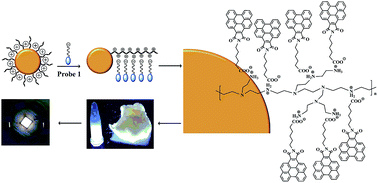
A bright white light emitting benzoperylene probe-polyethyleneimine capped quantum dot nanocomposite has been synthesized for the first time. The negatively charged benzoperylene probe with a carboxylic acid functional group tends to self-assemble in the presence of a polycation, and exhibits distinct induced excimer fluorescence. Phosphorescence emitting manganese doped zinc sulfide quantum dots (Mn-ZnS QDs) were capped with polyethyleneimine (PEI). The PEI capped Mn-ZnS QDs were used to induce the benzoperylene probe self-assembly. A bright white light emitting nanocomposite was obtained both in solution and in solid state. The white emission has Commission Internationale de l'Eclairage (CIE) coordinates of (0.33, 0.32), a color-rendering index (CRI) of 72.61 and correlated color temperature (CCT) of 5697 K. Multiple color emissions from blue to orange could also be obtained in a controllable manner via simple adjustment of the ratio of the PEI capped Mn-ZnS QDs and the benzoperylene probe. The nanocomposite displays good photo, chemical and long-term stability. A strong white light emitting LED device based on the nanocomposite was demonstrated.

 Please wait while we load your content...
Please wait while we load your content...