Antihyperglycemic and antihyperlipidemic activities of protodioscin in a high-fat diet and streptozotocin-induced diabetic rats†
Changrun Guo*ab,
Can Lib,
Yue Yub,
Wei Chenb,
Teng Mab and
Zhangjin Zhoub
aState Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing 210009, P. R. China. E-mail: crguocpu@126.com; Fax: +86 25 83271425; Tel: +86 25 83271425
bSchool of Chinese Medicines, China Pharmaceutical University, Nanjing 211198, P. R. China
First published on 12th September 2016
Abstract
Diabetes mellitus is one of the major health problems all over the world. The aim of this study was to investigate potential hypoglycemic and hypolipidemic effects of protodioscin in diabetic rats. After 4 weeks of feeding on a high-fat diet, rats were injected intraperitoneally with streptozotocin to induce diabetes. Diabetic rats were divided into 5 groups to receive carboxymethylcellulose sodium, metformin (200 mg kg−1), and 3 doses of protodioscin (10, 20 and 40 mg kg−1) continuously for 12 weeks. Blood levels of glucose, glycogen, total cholesterol (TC), triglycerides (TG), non-esterified fatty acids (NEFA), high density lipoprotein cholesterol (HDL-c), low density lipoprotein cholesterol (LDL-c), glycosylated hemoglobin (GHb), insulin, and adiponectin were measured, and glucose tolerance was estimated. The results demonstrated that protodioscin significantly improved glucose tolerance, reduced blood levels of glucose, GHb, TG, TC, LDL-c, and NEFA, as well as increased the content of HDL-c, adiponectin, and glycogen. Histological examination showed that protodioscin ameliorated the disordered liver structure in diabetic rats. Taken together, protodioscin alleviated hyperglycemia and hyperlipidemia in diabetic rats, and thus it can be considered a potential drug candidate for diabetes.
Introduction
Diabetes mellitus (DM), a common health problem worldwide, is a metabolic syndrome with multiple manifestations including hyperglycemia and dyslipidemia.1 Globally, the prevalence of DM is continually increasing due to the excessive intake of carbohydrates and lipids in the diet. The international Diabetes Federation2 estimated that the number of diabetic patients in the world would increase from 366 million in 2011 to 552 million by the year 2030. Currently, tens of millions of people suffer disabling and life-threatening complications of diabetes like kidney failure, heart attack, stroke, blindness, and amputation, and over four million die from DM every year.2 Hyperlipidemia, often caused by overeating and physical inactivity, is a risk factor for DM.1 Therefore, ideal therapeutic drugs of DM should not only have antihyperglycemic activities, but also favorable effects on hyperlipidemia. However, most of the antidiabetic drugs do not have favorable effects on the lipid profile.1 Therefore, it is necessary to search for antidiabetic drugs or functional foods, which can control hyperlipidemia as well as hyperglycemia.Members of the genus Dioscorea, such as Dioscoreae rhizome, Dioscoreae hypoglaucae rhizome, and Dioscoreae nipponicae rhizome, have been widely used for centuries as traditional medicines and foods in China and other countries. Dioscoreae rhizome (a rhizome of the genus Dioscorea opposita Thunb., ShanYao in Chinese) is a popular food in China, and has been widely used in traditional Chinese medicine for invigorating the spleen, stomach, and kidney.3 Pharmacological studies have demonstrated the therapeutic effects of Dioscoreae rhizome in numerous diseases, such as cardiovascular diseases, diabetes, and menopausal symptoms.4,5 The rhizome of Dioscoreae hypoglaucae (FenBiXie in Chinese), is another important traditional medicine in China used for removing dampness and descending turbid (State Pharmacopeia Committee of China). Pharmacological and clinical studies have revealed that Dioscoreae hypoglaucae rhizome has various pharmacological activities, such as antioxidant, antihyperglycemic, and antihyperlipidemic properties.6 The rhizome of Dioscorea nipponica Makino (ChanShanLong in Chinese) has been used as a herbal medicine or food supplement for thousands of years in China to improve blood circulation, relieve cough and asthma, eliminate rheumatism and alleviate pain.7 Protodioscin (Pdio, Fig. 1), a major steroidal saponin belonging to the genus Dioscorea, has been shown to exhibit multiple biological actions, such as anticancer, sexual, and cardiovascular properties.8–10 Pdio is the major ingredien of DiAoXinXueKang, which is the most commonly prescribed drug in China. Long term toxicity study showed that Di'AoXinXueKang was safe without toxic effect.11 It indicated that Pdio had good security.
Regarding the pathogenesis of DM and the pharmacological actions of Pdio, we hypothesized that Pdio has beneficial effects on hyperglycemia and hyperlipidemia. Thus, the aim of the present study was to analyze the effects of Pdio on DM. In this study, we evaluated the effects of Pdio on blood glucose, lipid profiles, and insulin sensitivity in a rat model of high-fat diet combined with low-dose streptozotocin (STZ)-induced diabetes.
Experimental
Reagents
Pdio (96.2% purity) was supplied by Spring Autumn Biological Engineering Co., Ltd, Nanjing, China. Metformin hydrochloride (Met), the positive control used in the experiments, was obtained from Beijing Zhonghui pharmaceutical Co., Ltd. Pdio and Met were suspended in 0.4% carboxymethylcellulose sodium (CMC-Na) solution for the animal model. STZ was purchased from Sigma-Aldrich Inc., Saint Louis, USA. Kits for blood glucose, glycogen, total cholesterol (TC), triglycerides (TG), non-esterified fatty acids (NEFA), high density lipoprotein cholesterol (HDL-c), low density lipoprotein cholesterol (LDL-c), and glycosylated hemoglobin (GHb) were purchased from Jiancheng Institute, Nanjing, China. Enzyme-linked immunosorbent assay (ELISA) kits for insulin, glucose transporter 4 (GLUT-4) and adiponectin were obtained from Cusabio Biotech Co., Ltd, Wuhan, China.Animals
Adult male SD rats (180–200 g) were purchased from Jiangsu University Laboratory Animal Center and kept at a maintained environment (temperature of 23 ± 2 °C, humidity of 50 ± 10%, and 12 h light/dark cycles) with access to normal laboratory food and water. All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals, and were approved by the ethics committee of China Pharmaceutical University (approval number: AECCPU20150809).Experimental design and induction of diabetes in rats
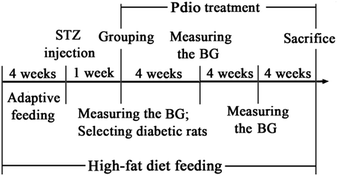
After habituation for 7 days, the animals were randomly divided into either the normal control (NC) or experimental group. The NC group received drinking water and standard chow, while the experimental group received drinking water and high-fat diet (consisting of 70% standard laboratory chow, 15% carbohydrates, 10% lard, and 5% yolk powder).12 After 4 weeks of high-fat diet feeding, the experimental group rats were injected with STZ (35 mg kg−1, dissolved in 0.01 M sodium citrate buffer, pH 4.4) intraperitoneally, while the NC group rats were injected with the equivalent amount of citrate buffer. Seven days after injection, rats with blood glucose level ≥ 16.7 mmol L−1 were considered diabetic and selected for further pharmacological studies. All rats were fed their respective diets until the end of the study.The diabetic rats were divided into 5 subgroups: diabetic control (DC) group (administered CMC-Na in a matched volume), Met group (administered 200 mg kg−1 Met), Pdio-10 group (administered 10 mg kg−1 Pdio), Pdio-20 group (administered 20 mg kg−1 Pdio), and Pdio-40 group (administered 40 mg kg−1 Pdio). Dose of Pdio was selected according to our preliminary experiment, other reports, and the clinical adult dose of D. nipponica rhizome.7,8 According to the pharmacopoeia of China, the highest dose of D. nipponica rhizome for human is 15 g per day. Equivalently, the calculated dose of D. nipponica rhizome based on respective body surface areas for rats is 1.34 g per kg per day. The highest content of Pdio in D. nipponica rhizome is 2.71%,7 and so the dose of Pdio for rats is 36.3 mg per kg per day. Therefore, we chose 40 mg per kg per day as high dose, 20 mg per kg per day as middle dose, and 10 mg per kg per day as low dose in this study. All drugs were administered orally once daily between 9:00 and 11:00 a.m., continuously for 12 weeks. Body weight was measured every 2 weeks, and blood glucose was measured every 4 weeks. The induction and treatment schedule is shown in Fig. 2.
Glucose tolerance
Glucose tolerance was estimated by an oral glucose tolerance test (OGTT) at the end of the treatment. After 12 h of fasting, rats were administered 2 g kg−1 glucose orally and blood samples were collected from the caudal vein following glucose administration by 0, 0.5, 1, and 2 h to measure the blood glucose levels. Results were expressed as the integrated area under glucose concentration time curve (AUC).Urine, blood, and tissue processing
At the end of the experiment, rats were housed in individual metabolic cages for 24 h to collect urine samples. Thereafter, blood samples were collected via the abdominal aorta and centrifuged at 4000 × g for 10 min at 4 °C to separate the plasma. After anaesthetization, rats' livers and gastrocnemius muscles were immediately harvested and weighed. The left lobes of the livers and the gastrocnemius muscles were rinsed with cold isotonic saline and subsequently stored at −80 °C until assayed, while the right lobes of the livers were washed in ice-cold saline solution and fixed in 4% neutral formaldehyde solution for histological examination.Analysis of blood biochemical parameters
To evaluate the hypoglycemic effect of Pdio, blood glucose and GHb levels were determined using a commercial diagnostic kit as previously reported.12 Serum levels of TG, TC, LDL-c, HDL-c, and NEFA were analyzed spectrophotometrically using a commercial diagnostic kit to estimate the hypolipidemic effects of Pdio. Serum insulin and adiponectin levels were assayed by ELISA kits according to the manufacturer's instructions.Amelioration of insulin resistance is one of the most important therapeutic approaches for type 2 diabetes mellitus (T2DM). To estimate the effect of Pdio on insulin resistance, homeostatic model assessment (HOMA) was calculated by the HOMA2 Calculator.12,13
Estimation of liver and muscle glycogen levels
Glycogen levels in the liver and skeletal muscles were assayed by the anthrone method according to a previous report,14 and were expressed as milligrams of glycogen per wet weight.Histological examination of the livers
After fixation in 4% neutral formaldehyde solution for 24 h at room temperature, the right lobes of the livers were dehydrated through a graded alcohol series, embedded in paraffin, and cut into 4 μm-thick sections. The sections were stained with hematoxylin-eosin (HE) and the related indicators were examined under a light microscope. Histopathological changes were graded as absent (0), mild (1), medium (2), and severe (3) according to the severity of changes in both remaining sections.Measurement of GLUT-4 content
Gastrocnemius muscle was thawed and homogenized with 5 mmol L−1 Tris–HCl. Muscle homogenate was centrifuged at 4000 × g for 10 min. The GLUT-4 content in supernatants was immediately assayed by ELISA kits according to the manufacturer's instructions.Statistical analysis
All results were expressed as mean ± standard deviation (n = 10/group). Data sets with multiple comparisons were evaluated with one-way analysis of variance (ANOVA), followed by a post hoc test (Dunnett's test). In all cases, differences were considered significant at probability values of p < 0.05.Results and discussion
Effects of Pdio on body weight
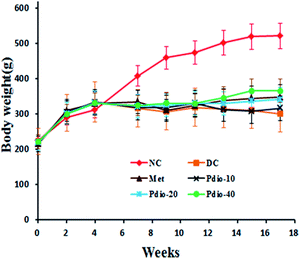
Body weights of NC rats continued to increase during the entire study (from 222 ± 24 g to 526 ± 42 g). Compared with the NC group, the high-fat diet did not significantly alter the rats' body weights during the first 4 weeks, however, body weights of DC rats were significantly lower (p < 0.05) than the NC group after STZ injection (Fig. 3). During the last 4 weeks, Pdio (40 mg kg−1) significantly restored the STZ-induced decrease in body weight (p < 0.05).Effects of Pdio on food intake, water intake, and urine volume
As shown in Table 1, food intake, water intake, and urine volumes of the DC group were significantly higher than the NC group (p < 0.01). After Pdio treatment, water intake and urine volumes decreased significantly (p < 0.01). Moreover, Pdio significantly decreased food consumption (20 mg kg−1, p < 0.05; 40 mg kg−1, p < 0.01). These results indicate that Pdio treatments are indicative of improvement of diabetic conditions.| Groups | Food intake (g kg−1 bw) | Water intake (mL kg−1 bw) | Urine volume (mL kg−1 bw) |
|---|---|---|---|
| a The data are expressed as the means ± SD (n = 10). ΔΔp < 0.01 compared with the NC group; #p < 0.05, ##p < 0.01 compared with the DC group. | |||
| NC | 74.5 ± 9.7 | 79.0 ± 10.3 | 83.9 ± 16.3 |
| DC | 173.0 ± 22.5ΔΔ | 550.4 ± 71.5ΔΔ | 467.5 ± 59.8ΔΔ |
| Met | 122.2 ± 15.9## | 249.5 ± 32.4## | 218.8 ± 34.4## |
| Pdio-10 | 151.3 ± 19.7 | 254.6 ± 33.1## | 213.7 ± 22.4## |
| Pdio-20 | 128.7 ± 16.7# | 224.0 ± 42.1## | 202.5 ± 20.1## |
| Pdio-40 | 106.1 ± 13.8## | 209.6 ± 27.2## | 193.6 ± 32.4## |
Effects of Pdio on OGTT
Compared with the NC group, the DC group had significantly increased blood glucose levels following the oral administration of 2 g kg−1 glucose by 0, 0.5, 1, and 2 h (p < 0.01). Likewise, the AUC of blood glucose in DC rats increased significantly (p < 0.01). After treatment with Pdio for 12 weeks, blood glucose levels and AUC (10 mg kg−1, p < 0.05; 20 and 40 mg kg−1, p < 0.01) were significantly reduced (Fig. 4). These results indicate that Pdio treatment can effectively attenuate high-fat diet and STZ-induced glucose intolerance. | ||
| Fig. 4 Effects of Pdio on OGTT in diabetic rats. The data are expressed as the means ± SD (n = 10). ΔΔp < 0.01 compared with the NC group; #p < 0.05, ##p < 0.01 compared with the DC group. | ||
Effects of Pdio on blood glucose, insulin, and HOMA value
To evaluate the hypoglycemic effect of Pdio, blood glucose levels were measured at weeks 0, 4, 8, and 12 of the treatment. Compared with NC rats, blood glucose levels of DC rats were increased significantly (p < 0.01) after the STZ injection (Table 2) and before the administration of the respective drugs, while the blood glucose levels of the five diabetic subgroups (DC group, Met group, Pdio-10 group, Pdio-20 group, and Pdio-40 group) were almost identical. Treatment with Pdio significantly decreased the blood glucose levels at weeks 4 (10 and 20 mg kg−1, p < 0.05; 40 mg kg−1, p < 0.01), 8 (10 mg kg−1, p < 0.05; 20 and 40 mg kg−1, p < 0.01) and 12 (10, 20 and 40 mg kg−1, p < 0.01).| Groups | Blood glucose (mmol L−1) | Insulin (mU L−1) | HOMA value | |||
|---|---|---|---|---|---|---|
| Before treatment | 4 weeks treatment | 8 weeks treatment | 12 weeks treatment | |||
| a The data are expressed as the means ± SD (n = 10). ΔΔp < 0.01 compared with the NC group; #p < 0.05, ##p < 0.01 compared with the DC group. | ||||||
| NC | 5.2 ± 0.8 | 6.5 ± 1.4 | 6.4 ± 0.5 | 6.7 ± 0.9 | 21.2 ± 1.7 | 6.3 ± 0.4 |
| DC | 26.4 ± 4.3ΔΔ | 28.6 ± 2.9ΔΔ | 28.5 ± 3.7ΔΔ | 26.6 ± 2.6ΔΔ | 14.3 ± 1.3ΔΔ | 16.9 ± 2.5ΔΔ |
| Met | 25.3 ± 5.1 | 20.6 ± 3.8## | 17.8 ± 4.7## | 18.8 ± 4.9# | 15.9 ± 1.4 | 13.1 ± 3.0 |
| Pdio-10 | 25.4 ± 3.5 | 21.1 ± 4.2# | 19.5 ± 4.8# | 17.9 ± 3.6## | 15.5 ± 2.5 | 12.6 ± 4.2 |
| Pdio-20 | 25.3 ± 3.7 | 20.2 ± 4.6# | 17.7 ± 3.5## | 17.0 ± 3.9## | 15.0 ± 3.0 | 11.3 ± 3.1# |
| Pdio-40 | 25.1 ± 2.5 | 18.4 ± 4.0## | 17.0 ± 4.4## | 15.4 ± 3.3## | 15.1 ± 2.1 | 10.3 ± 2.1## |
As expected, serum levels of insulin significantly decreased in the DC group (Table 2). Pdio did not affect serum insulin levels in diabetic rats, but Pdio treatment markedly reduced HOMA values compared with the DC group (20 mg kg−1, p < 0.05; 40 mg kg−1, p < 0.01). These results suggest that Pdio ameliorates insulin resistance induced by high-fat diet and STZ.
Effect of Pdio on GHb
The effect of Pdio on GHb is shown in Fig. 5. GHb levels in DC rats were 24% higher than the index of the NC rats (p < 0.01). However, treatment with Pdio significantly reversed the increase in the GHb levels (10 mg kg−1, p < 0.05; 20 and 40 mg kg−1, p < 0.01). | ||
| Fig. 5 Effects of Pdio on GHb in diabetic rats. The data are expressed as the means ± SD (n = 10). ΔΔp < 0.01 compared with the NC group; #p < 0.05, ##p < 0.01 compared with the DC group. | ||
Effects of Pdio on liver and muscle glycogen content
As shown in Table 3, high-fat diet and STZ markedly reduced liver and muscle glycogen contents. However, treatment with Pdio significantly restored the liver glycogen (20, 40 mg kg−1, p < 0.05) and muscle glycogen (10 and 20 mg kg−1, p < 0.05; 40 mg kg−1, p < 0.01) content.| Groups | Liver index (g kg−1 bw) | Liver glycogen (mg g−1 liver) | Muscle glycogen (mg g−1 muscule) |
|---|---|---|---|
| a The data are expressed as the means ± SD (n = 10). ΔΔp < 0.01 compared with the NC group; #p < 0.05, ##p < 0.01 compared with the DC group. | |||
| NC | 23.2 ± 2.4 | 13.27 ± 2.88 | 1.92 ± 0.27 |
| DC | 45.9 ± 5.2ΔΔ | 9.16 ± 0.95ΔΔ | 1.37 ± 0.16ΔΔ |
| Met | 42.0 ± 13.2 | 12.54 ± 2.70# | 1.76 ± 0.46# |
| Pdio-10 | 43.3 ± 6.4 | 10.87 ± 2.06 | 1.66 ± 0.29# |
| Pdio-20 | 37.8 ± 6.0# | 11.37 ± 1.43# | 1.65 ± 0.18# |
| Pdio-40 | 35.9 ± 5.8# | 11.84 ± 2.08# | 1.82 ± 0.22## |
Effects of Pdio on the lipid profile
As shown in Table 4, high-fat diet and STZ induced dyslipidemia in rats, characterized by a significant increase in TC, TG, NEFA, and LDL-c (p < 0.01) levels and a significant decrease in HDL-c (p < 0.01) level. Treatment with Pdio markedly reduced the serum levels of TC (10, 20 and 40 mg kg−1, p < 0.01), TG (10, 20 and 40 mg kg−1, p < 0.01), LDL-c (10 mg kg−1, p < 0.05; 20 and 40 mg kg−1, p < 0.01), and NEFA (10 and 20 mg kg−1, p < 0.05; 40 mg kg−1, p < 0.01) in diabetic rats. Furthermore, Pdio significantly attenuated the decrease in serum HDL-c levels when compared with DC rats (10, 20 and 40 mg kg−1 p < 0.01). These results indicate that Pdio treatment can effectively attenuate high-fat diet and STZ induced dyslipidemia.| Groups | TG (mmol L−1) | TC (mmol L−1) | LDL-c (mmol L−1) | NEFA (μmol L−1) | HDL-c (mmol L−1) |
|---|---|---|---|---|---|
| a The data are expressed as the means ± SD (n = 10). ΔΔp < 0.01 compared with the NC group; #p < 0.05, ##p < 0.01 compared with the DC group. | |||||
| NC | 0.71 ± 0.26 | 2.90 ± 0.38 | 1.45 ± 0.14 | 321 ± 46 | 1.56 ± 0.38 |
| DC | 2.33 ± 0.38ΔΔ | 5.39 ± 0.77ΔΔ | 3.80 ± 1.27ΔΔ | 429 ± 49ΔΔ | 0.85 ± 0.09ΔΔ |
| Met | 1.41 ± 0.33## | 4.38 ± 0.95 | 2.53 ± 0.59 | 358 ± 61 | 1.08 ± 0.26# |
| Pdio-10 | 1.50 ± 0.33## | 3.54 ± 0.75## | 2.21 ± 0.55# | 351 ± 64# | 1.22 ± 0.27## |
| Pdio-20 | 1.38 ± 0.36## | 3.51 ± 0.61## | 2.07 ± 0.62## | 359 ± 50# | 1.25 ± 0.39## |
| Pdio-40 | 1.23 ± 0.29## | 3.22 ± 1.12## | 1.69 ± 0.92## | 327 ± 66## | 1.54 ± 0.31## |
Effects of Pdio on liver histology and liver index
Liver sections from the control rats showed normal histology (Fig. 6A). However, high-fat diet and STZ induced pathological changes in the hepatic cells and hepatic architecture of diabetic rats. Fig. 6A shows the disordered liver structure with hepatocellular necrosis and extensive vacuolization in the diabetic rats. Pdio treatment for 12 weeks significantly ameliorated these changes (Fig. 6A and B). In addition, high-fat diet and STZ markedly increased (p < 0.01) the liver index (Table 3). However, treatment with Pdio significantly decreased the liver index (20, 40 mg kg−1, p < 0.05).Effects of Pdio on serum adiponectin content
Adiponectin play a critical role in the development of insulin resistance. As shown in Fig. 7, the adiponectin levels of DC group and NC group were quite similar, while Pdio treatment significantly increased the adiponectin content of diabetic rats (20 and 40 mg kg−1, p < 0.05). | ||
| Fig. 7 Effect of Pdio on adiponectin in diabetic rats. The data are expressed as the means ± SD (n = 10). #p < 0.05 compared with the DC group. | ||
Effects of Pdio on GLUT-4 content in muscle
As shown in Fig. 8, high-fat diet and STZ markedly reduced GLUT-4 contents (p < 0.01). However, treatment with Pdio significantly restored the GLUT-4 content in muscle (20, 40 mg kg−1, p < 0.05). | ||
| Fig. 8 Effect of Pdio on GLUT-4 in diabetic rats. The data are expressed as the means ± SD (n = 10). ΔΔp < 0.01 compared with the NC group; #p < 0.05 compared with the DC group. | ||
Discussion
Many studies have reported the rat model of high-fat diet combined with low doses of STZ-induced diabetes as one of the animal models of human diabetes mellitus.15,16 This model has the characteristics of late-stage T2DM, including impaired insulin secretion, hyperglycemia, insulin resistance, dyslipidemia, polydipsia, polyphagia, polyuria, and emaciation.17 In the present study, we fed rats a high-fat diet for 4 weeks and injected them with low doses of STZ (30 mg kg−1) to mimic T2DM, and then investigated the antihyperglycemic and antihyperlipidemic activities of Pdio in this model. We found that Pdio treatment attenuated high-fat diet and STZ-induced body weight loss, polyphagia, polydipsia, and polyuria.Hyperglycemia is the main cause of diabetic complications, including diabetic nephropathy, diabetic ophthalmopathy, diabetic foot, and diabetic cardiopathy. Therefore, correcting hyperglycemia is considered as the primary therapeutic goal of diabetes. Fasting glucose and GHb levels are both classic assays of glucose. Fasting glucose has high intra-individual variability and it does reflect an immediate response to stress,18 while GHb reflects the 3-month average blood glucose level.19 In the present study, treatment with Pdio significantly reduced the GHb level in diabetic rats, which provided direct evidence for the hypoglycemic effect of Pdio. Diabetes generally causes increases of food intake, water intake, and urine volume,20 reductions of food intake, water intake, and urine volume after Pdio treatments are indicative of improvement of diabetic conditions.
Generally, deficient insulin secretion and insulin resistance are the primary causes of diabetes. Therefore, we chose a rat model feeding on a high fat-diet and injected with STZ as the experimental animal model, in order to mimic the pathogenesis and clinical features of human T2DM.21 Our results showed that high fat-diet and STZ injection significantly damage the pancreatic islets (data not shown) and decrease insulin release. Fallaciously, treatment with Pdio did not ameliorate the islets damage or promote insulin secretion. Thus, we inferred that Pdio might enhance the sensitivity of target tissues to insulin. Therefore, the HOMA value, which reflects the extent of insulin resistance in diabetes, was determined in this study. Our results showed that HOMA value was significantly increased in diabetic rats, whereas treatment with Pdio significantly lowered it. Those results suggest that Pdio reduced blood glucose levels in high-fat diet and STZ-induced diabetic rats by improving insulin sensitivity rather than stimulating insulin release.
Previous studies have demonstrated that lipid abnormalities play a significant role in the development of diabetes and its complications.1,21 High levels of TG and NEFA are the main causes of both peripheral and hepatic insulin resistance. Oxidation of TG and NEFA competitively inhibits glucose oxidation, and thus reduces glucose uptake and utilization in skeletal muscles and leads to insulin resistance. Furthermore, lipid abnormalities impair insulin secretion and action leading to β-cell failure.22 On the other hand, insulin deficiency aggravates dyslipidemia abnormalities in lipid metabolism.23 In our study, treatment with Pdio inhibited lipid abnormalities in diabetic rats (as evidenced by decreased TG, NEFA, and LDL-c).
The liver has a regulatory role in carbohydrate and fat metabolism. It is well known that the liver stores glucose as glycogen and hydrolyzes glycogen to glucose to maintain normal blood glucose levels. Hyperglycemia and hyperlipidemia have deleterious effects on hepatic cells, the phenomena termed glucotoxicity and lipotoxicity. In this study, we induced fat accumulation and lipogenesis in the liver, however, these abnormal changes were efficiently attenuated by Pdio treatment.
Adipocytes play a critical role in the development of insulin resistance. They abundantly express several adipokines, including adiponectin. Adiponectin binds to its receptors to regulate lipid/glucose metabolism and is considered as a novel pharmacological target for the treatment of T2DM.24 At least two functions of adiponectin were confirmed: first, adiponectin suppresses lipogenesis and gluconeogenesis via increasing the phosphorylation of adenosine monophosphate (AMP)-activated protein kinase; second, it increases fatty acid oxidation and energy consumption via activating peroxisome proliferator-activated receptor α ligand.25,26 In the present study, we showed that Pdio increased adiponectin protein concentration.
GLUT-4 plays a critical role in the regulation of glucose homeostasis through the translocation and activation triggered by insulin.27 Intracellular GLUT-4 translocates to the plasma membrane and facilitates glucose uptake stimulated by insulin. Under the diabetic condition, GLUT-4 expression reduced due to the impairment of insulin signaling. In the present study, we showed that Pdio increased GLUT-4 content in muscle.
Conclusions
In summary, the present study demonstrated for the first time that Pdio attenuated the increase in blood glucose, GHb, lipid levels, urine volumes, and water and food consumption induced by high fat-diet and STZ in rats. The mechanism by which it does so might be improvement of insulin sensitivity and increasing adiponectin concentrations, at least in part. Further studies will be in progress to elucidate the exact mechanism(s) of Pdio's antihyperglycemic and antihyperlipidemic activities.Acknowledgements
This work was supported by a project funded by the College Students Innovation Project for the R&D of Novel Drugs (No. J1030830). The assistance of the staff is gratefully acknowledged.Notes and references
- A. Aissaoui, S. Zizi, Z. H. Israili and B. Lyoussi, J. Ethnopharmacol., 2011, 137, 652–661 CrossRef PubMed.
- InternationalDiabetesFederation, global diabetes plan 2011–2012., 2011, p. 4 Search PubMed.
- W. Shujun, G. Wenyuan, L. Hongyan, C. Haixia, Y. Jiugao and X. Peigen, Food Chem., 2006, 99, 38–44 CrossRef.
- S. C. Lee, C. C. Tsai, J. C. Chen, C. C. Lin, M. L. Hu and S. Lu, Am. J. Chin. Med., 2002, 30, 609–616 CrossRef PubMed.
- M. A. McAnuff-Harding, F. O. Omoruyi and H. N. Asemota, Life Sci., 2006, 78, 2595–2600 CrossRef CAS PubMed.
- C. Guo, G. Ding, W. Huang, Z. Wang, Z. Meng and X. Wei, Drug Des., Dev. Ther., 2016, 2016, 799–810 Search PubMed.
- T. Wang, R. C. Choi, J. Li, J. Li, C. W. Bi, L. Zang, Z. Liu, T. T. Dong, K. Bi and K. W. Tsim, Planta Med., 2010, 76, 1642–1646 CrossRef CAS PubMed.
- K. Gauthaman, A. P. Ganesan and R. N. Prasad, J. Altern. Complement. Med., 2003, 9, 257–265 CrossRef PubMed.
- X. He, A. Qiao, X. Wang, B. Liu, M. Jiang, L. Su and X. Yao, Steroids, 2006, 71, 828–833 CrossRef CAS PubMed.
- K. Hu and X. Yao, Planta Med., 2002, 68, 297–301 CrossRef CAS PubMed.
- X. Z. Zheng, F. Ni, H. Y. Cheng, X. M. Xu and B. Y. Wang, New Drugs Clin. Rem., 1995, 14, 354–355 Search PubMed.
- C. Guo, C. Zhang, L. Li, Z. Wang, W. Xiao and Z. Yang, Phytomedicine, 2014, 21, 807–814 CrossRef CAS PubMed.
- T. M. Wallace, J. C. Levy and D. R. Matthews, Diabetes Care, 2004, 27, 1487–1495 CrossRef PubMed.
- N. V. Carroll, R. W. Longley and J. H. Roe, J. Biol. Chem., 1956, 583–593 CAS.
- C. Zhang, G. Xu, Y. Liu, Y. Rao, R. Yu, Z. Zhang, Y. Wang and L. Tao, Phytomedicine, 2013, 20, 221–229 CrossRef CAS PubMed.
- R. Sundaram, R. Naresh, P. Shanthi and P. Sachdanandam, Phytomedicine, 2013, 20, 577–584 CrossRef CAS PubMed.
- K. Srinivasan, B. Viswanad, L. Asrat, C. L. Kaul and P. Ramarao, Pharmacol. Res., 2005, 52, 313–320 CrossRef CAS PubMed.
- C. Liu, C. Yang, Y. Zhao, Z. Ma, J. Bi, Y. Liu, X. Meng, Y. Wang, J. Cai, H. Kan and R. Chen, Environ. Int., 2016, 92–93, 416–421 CrossRef CAS PubMed.
- G. Pararajasingam, D. E. Høfsten, B. B. Løgstrup, M. Egstrup, F. L. Henriksen, J. Hangaard and K. Egstrup, Int. J. Cardiol., 2016, 214, 310–315 CrossRef PubMed.
- Y. Wang, T. Campbell, B. Perry, C. Beaurepaire and L. Qin, Metabolism, 2011, 60, 298–305 CrossRef CAS PubMed.
- W. Li, M. Zhang, J. Gu, Z. Meng, L. Zhao, Y. Zheng, L. Chen and G. Yang, Fitoterapia, 2012, 83, 192–198 CrossRef CAS PubMed.
- K. Cao, J. Xu, X. Zou, Y. Li, C. Chen, A. Zheng, H. Li, H. Li, I. M. Szeto, Y. Shi, J. Long, J. Liu and Z. Feng, Free Radical Biol. Med., 2014, 67, 396–407 CrossRef CAS PubMed.
- S. S. Moree, G. B. Kavishankar and J. Rajesha, Phytomedicine, 2013, 20, 237–245 CrossRef CAS PubMed.
- Z. Fu, Y. Gong, C. Löfqvist, A. Hellström and L. E. H. Smith, Biochim. Biophys. Acta, Mol. Basis Dis., 2016, 1862, 1392–1400 CrossRef CAS PubMed.
- T. Yamauchi, Y. Nio, T. Maki, M. Kobayashi, T. Takazawa, M. Iwabu, M. Okada-Iwabu, S. Kawamoto, N. Kubota, T. Kubota, Y. Ito, J. Kamon, A. Tsuchida, K. Kumagai, H. Kozono, Y. Hada, H. Ogata, K. Tokuyama, M. Tsunoda, T. Ide, K. Murakami, M. Awazawa, I. Takamoto, P. Froguel, K. Hara, K. Tobe, R. Nagai, K. Ueki and T. Kadowaki, Nat. Med., 2007, 13, 332–339 CrossRef CAS PubMed.
- T. Yamauchi and T. Kadowaki, Cell Metab., 2013, 17, 185–196 CrossRef CAS PubMed.
- H. Y. Kim and K. Kim, J. Ethnopharmacol., 2012, 142, 53–58 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18448k |
| This journal is © The Royal Society of Chemistry 2016 |