Hydrolysis behaviors of sugarcane bagasse pith in subcritical carbon dioxide–water
Jiezhen Liangab,
Xiaopeng Chen*ab,
Linlin Wangab,
Xiaojie Weiab,
Feifei Qiua and
Chaochao Lua
aSchool of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, P. R. China. E-mail: lilm@gxu.edu.cn
bKey Laboratory for the Petrochemical Resources Processing and Process Intensification Technology of Guangxi, Nanning, 530004, P. R. China
First published on 11th October 2016
Abstract
The aim of this study was to describe the hydrolysis behavior of sugarcane bagasse pith (SCBP) in subcritical CO2–water. The hydrolysis was carried out in a batch reactor using different temperatures (160 to 260 °C), liquid to solid ratios (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 100
1 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), CO2 pressures (0 to 7.3 MPa), stirring speeds (0 to 500 rpm) and reaction times (0 to 40 min). The highest total reducing sugar yield (43.6%) was obtained at 200 °C, liquid to solid ratio 30
1), CO2 pressures (0 to 7.3 MPa), stirring speeds (0 to 500 rpm) and reaction times (0 to 40 min). The highest total reducing sugar yield (43.6%) was obtained at 200 °C, liquid to solid ratio 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 MPa CO2, 500 rpm and 50 min. Two-dimensional heteronuclear single quantum coherence (2D HSQC) nuclear magnetic resonance (NMR), scanning electron microscopy (SEM) and Fourier transform infrared spectrometry (FT-IR) were used to help elucidate the physical and chemical characteristics of the raw material and residual solid particles, with results consistent with the removal of hemicellulose during hydrolysis. The changes in the concentration of products with time were analyzed to understand product distribution through high-performance liquid chromatography (HPLC) and to infer the reaction mechanism.
1, 2 MPa CO2, 500 rpm and 50 min. Two-dimensional heteronuclear single quantum coherence (2D HSQC) nuclear magnetic resonance (NMR), scanning electron microscopy (SEM) and Fourier transform infrared spectrometry (FT-IR) were used to help elucidate the physical and chemical characteristics of the raw material and residual solid particles, with results consistent with the removal of hemicellulose during hydrolysis. The changes in the concentration of products with time were analyzed to understand product distribution through high-performance liquid chromatography (HPLC) and to infer the reaction mechanism.
1 Introduction
Lignocellulose biomass is a complex polymer composed of three main fractions: cellulose, hemicellulose and lignin.1 Lignocellulose biomass, an agricultural waste product, is considered as a potential feedstock for producing fuels, cellulosic pulp, biomaterials and a variety of chemicals because it is available in large quantities at low cost. One major lignocellulosic waste product is sugarcane bagasse.Sugarcane bagasse, the fibrous residue remaining after extracting the juice from sugarcane during the sugar production process, is mainly used in papermaking. About 40% of this residue is a smaller fibre, sugarcane bagasse pith (SCBP), which is removed during the process of producing pulp for papermaking to reduce paper brittleness. According to the Food and Agriculture Organization of the United Nations, about 523 million tons of bagasse was produced in 2014 throughout the world,2 given that one ton of sugarcane produces 280 kg of bagasse.3 Only 40% of the bagasse is used in the paper industry, so the rest (including bagasse pith) is burnt to produce steam and electricity.4 Therefore, because of the importance of SCBP as an industrial waste, there is great interest in developing methods for the biological production of fuel and chemicals that offer economic, environmental, and strategic advantages. Compared with other agro-based residues such as rice straw, paddy straw, and wheat straw, SCBP also offers many advantages: it is available in relatively large quantities, centralised at sugar factories and comminuted during the sugar production process.
The bioconversion of lignocellulose to fuel and added-value chemicals requires an efficient hydrolysis process to produce fermentable sugars (reducing sugars) which should be free of inhibitors to provide the efficient fermentation.5 Pretreated lignocellulosic materials can be hydrolysed using acids or enzymes. However, using concentrated acids, such as H2SO4 and HCl,6 requires corrosion-resistant reactors. These acids are also toxic, corrosive and hazardous in nature. The major bottleneck to make enzymatic hydrolysis economically feasible is the long process time and the high cost of producing enzyme.7 Another optional process, subcritical water treatment, has been developed to be highly environmentally friendly. Some chemical reactions can occur in supercritical and subcritical water without causing environment pollution.
Subcritical water has been extensively investigated for the hydrolysis and conversion of lignocellulosics to produce useful compounds.8–12 Subcritical water can dissolve hemicellulose completely and partially remove lignin at high temperatures, such as 220 °C, within 2 min without the use of chemicals.13 During subcritical water hydrolysis, more than 80% of lignocellulosic biomass is hydrolysed to low-molecular-weight components, such as glucose, xylose and several phenolic compounds. Cellulose and hemicellulose are easily hydrolysed to monomeric sugars by subcritical water.14 Subcritical water hydrolysis has been used to produce reducing sugars from lignocellulosic biomass, such as Miscanthus giganteus,15 municipal solid waste,16 cornstalks,17 wheat straw,17 rice bran,18 and other solid wastes. Pressurised carbon dioxide has been used to acidify the medium through the formation of carbonic acid and to increase the efficiency of subcritical-water-mediated hydrolysis of biomass.19,20 However, scanty information is available on the hydrolysis of SCBP in subcritical water, particularly in combining with the use of CO2. In the present study, subcritical CO2–water hydrolysis will be used to produce reducing sugars from SCBP. The treated and untreated SCBP samples will be characterized using two-dimensional heteronuclear single quantum coherence nuclear magnetic resonance (2D HSQC NMR), Fourier transform infrared (FTIR) spectroscopy, and scanning electron microscopy (SEM) techniques to help elucidate the physical and chemical characteristics of the raw material and the residual solid particles. The possible reaction process will also be investigated.
2 Experimental
Materials
The SCBP used as a raw material in this study was obtained from a local mill (Nan-Ning Sugar Mill, Guang-Xi, China). The HPLC standards, xylose (>P98%), glucose (P99.5%), arabinose (P99%), cellobiose (P98%), cellotriose (P95%), cellotetraose (P94%), cellopentaose (>P95%), xylobiose (P98%), glyceraldehyde (>P90%), acetic acid (P99.9%), lactic acid (P90%), 5-(hydroxymethyl)furfural (5-HMF, P99%), furfural (P99.5%), and ethanol (P99.8%) were purchased from Toronto Research Chemicals (Toronto, Canada).Apparatus
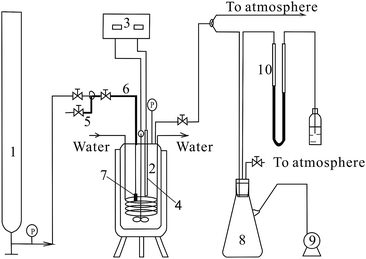
The hydrolysis of SCBP was carried out in high pressure reactor (design pressure up to 20.0 MPa and temperatures up to 350 °C, Dalian Jingyi Autoclave Vessel Manufacturing Co. Ltd., Dalian, China) equipped with a magnetic driven paddle agitator at the bottom, a temperature controller, a cooling coil, a heat exchanger, a CO2 inlet, and a sampling device. The reactor was heated with the help of 1.5 kW electric heaters. Considering that the hydrolysis liquor tends to crystallise, we used specific sampling device to overcome problems in a system at a high pressure and temperature. In particular, the samples were withdrawn by using a sampling tube extending almost to the bottom of the reactor. A stainless steel filter with apertures of less than 10 μm was fixed onto the bottom of the sampling tube prevented the residual solid particles from being withdrawn with the liquid sample. The stainless steel sampling tube (diameter = 3 mm) outside the reactor was insulated to prevent cooling and the solidification of the hydrolysis liquor. For sampling, the T-valve in the sample cell was opened, and the sampling cell was connected to the reactor. The samples for 3,5-dinitrosalicylic acid (DNS) and HPLC analysis were withdrawn at predetermined reaction times. The apparatus employed in the experiment is shown schematically in Fig. 1.Hydrolysis procedure
In each experimental run, a set amount of SCBP was mixed with 1000 mL of water in the reactor. The reactor was then closed and sealed tightly. The air was pumped out of the reactor to an absolute pressure of approximately 0.003 MPa then pressurised with carbon dioxide for 10 min. After pressurization, the temperature was increased to the desired level and maintained for a specified time. The influence of temperature (160, 180, 200, 220, 240 and 260 °C), liquid to solid ratio (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 30
1, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 60
1, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 80
1, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 100
1 and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), CO2 initial pressure (0, 1, 4, and 7.3 MPa), stirring speed of (100, 200, 400 and 500 rpm), and reaction time (1, 3, 5, 10, 20, 30, 40, 50 and 60 min) on the performance of hydrolysis of SCBP in subcritical CO2–water were investigated. The samples were withdrawn at predetermined reaction times for DNS and HPLC analysis. The power controller was switched off and the mixtures were naturally cooled to room temperature. The hydrolysis mixtures were separated in a vacuum into solid and liquid solutions through filtration. Solid residues were placed in an oven at 80 °C for 8 h to dry the samples. The samples were then analysed through scanning electron microscopy (SEM) and Fourier-transform infrared spectrometry (FTIR) to figure out their properties.
1), CO2 initial pressure (0, 1, 4, and 7.3 MPa), stirring speed of (100, 200, 400 and 500 rpm), and reaction time (1, 3, 5, 10, 20, 30, 40, 50 and 60 min) on the performance of hydrolysis of SCBP in subcritical CO2–water were investigated. The samples were withdrawn at predetermined reaction times for DNS and HPLC analysis. The power controller was switched off and the mixtures were naturally cooled to room temperature. The hydrolysis mixtures were separated in a vacuum into solid and liquid solutions through filtration. Solid residues were placed in an oven at 80 °C for 8 h to dry the samples. The samples were then analysed through scanning electron microscopy (SEM) and Fourier-transform infrared spectrometry (FTIR) to figure out their properties.
Analysis methods
The components of SCBP were measured according to the National Renewable Energy Laboratory (NREL) procedure.21 Briefly, SCBP (200 mg) was homogenized with 72% sulphuric acid (2 mL) at 30 °C for 2 h then heated for 1 h at 120 °C after dilution to 4% with distilled water. The monomeric sugars released from sulphuric acid hydrolysis of the raw SCBP were analyzed using HPLC (LC1220, Agilent, Santa Clara, CA, USA) equipped with a SUGAR KS-801 column (Shodex, Tokyo, Japan) and an RI detector (RID1200, Agilent).The total reducing sugar concentration was estimated by the 3,5-dinitro salicylic acid (DNS) method,22 with xylose used as a standard. The hydrolysis liquor was diluted 10 times. For each 1 mL of the diluted liquor, 1 mL DNS reagent and 1 mL deionized water were added. The mixture was then heated in boiling water for 10 min until the red brown color was developed. Then, the mixture was cooled to room temperature in a water bath and diluted to 25 mL. The absorbance was then measured using an ultraviolet-visible spectrophotometer (UV-2100, Unico, Dayton, NJ, USA) at 508 nm. The concentration of total reducing sugars was calculated based on a standard curve obtained with xylose.
The sugars, organic acids and other degradation products in the liquid samples were quantified by HPLC (LC1220, Agilent). Each sample was diluted 15-fold and then filtered through a 0.45 μm syringe filter. The filtered sample was injected into a SUGAR KS-801 (Shodex) column and eluted with water at a flow rate of 1.0 mL min−1 and a constant temperature of 80 °C. An RI detector (RID1200, Agilent) was used to provide a quantitative analysis of the products obtained from the hydrolysis process. The HPLC peaks were identified by comparing the sample peak retention time with the help of calibration plots for pure compounds.
The chemical changes in the SCBP before and after hydrolysis were investigated using FT-IR (Nicolet 380, Thermo Scientific, Waltham, MA, USA). The SCBP sample was mixed with spectroscopic grade dried KBr to create a disk. The spectra were collected in the spectral range 400–4000 cm−1 using 30 scans per sample. The background spectrum of pure dried KBr was subtracted from the sample spectrum.
The surface morphology of SCBP before and after hydrolysis was qualitatively studied using SEM (Hitachi S-3400N, Tokyo, Japan). The dried samples were fixed using a conducting adhesive tape to an aluminium stub and then sputter coated with gold for 120 s with a gold sputter coater (Polaron SC 7640, Quorum Technologies, Ashford, UK). The images of the gold coated samples were obtained in the SEM operating at 10 kV.
The 2D HSQC-NMR spectra (75 MHz) were recorded on a Nuclear Magnetic Resonance Spectrometer (AVANCE III HD600, Bruker, Flatlander, Switzerland) at room temperature. Dried samples (1 mg) were dissolved in sodium deuteroxide (DMSO-d6, 1.5 mL) then transferred to an NMR tube (diameter, 5 mm).
3 Results and discussion
Composition of the raw SCBP
The raw SCBP was composed of cellulose (30.16%), hemicellulose (40.01%), lignin (18.09%), moisture (9.41%), and ash (2.33%). It is clear that cellulose, hemicellulose and lignin are present in significant quantities in the raw SCBP while ash is present in low amounts compared with other agro-industrial residues such as paddy straw (16%),23 rice straw (14.5%)24 and wheat straw (9.2%).17 Because of its lower ash content, SCBP offers many advantages as a potential source of biomass for the production of reducing sugar.NMR results
To obtain information about the structure of hemicelluloses in SCBP, the HSQC-NMR spectroscopic analysis was performed. Fig. 2 showed 2D HSQC-NMR spectra of the SCBP. The assignments of signals are based on previous publications.25,26 As shown in Fig. 2, the signals observed at δC/δH 102.3/4.26, 72.2/3.23, 73.3/3.55, 77.7/3.67 and 63.6/3.37 were related to Cβ–Hβ correlations corresponding to C-1, C-2, C-3, C-4 and C-5 of the 1,4-linked D-xylose units, respectively. The signal at δC/δH 61.1/3.55 was related to C-5 of the α-L-arabinose residue. The signals at δC/δH 98.7/4.68, 71.6/3.44, and 72.9/3.64 were assigned to C-1, C-2 and C-3 of the glucuronic acid residue, respectively. The prominent signal for methoxyl (δC/δH 56.2/3.72) was probably a result of the 4-omethoxyl group of glucuronic acid residues in the hemicelluloses and aromatic methoxyl groups in the lignin. The weak signal at δC/δH 21.3/2.02 CH3 may be attributed to acetyl in the hemicelluloses.The main cross-signals observed in the aromatic region (δC/δH 100.0–135.0/5.50–8.50) were assigned to the p-hydroxyphenyl, guaiacyl and syringyl units of lignin, respectively. The signal at δC/δH 104.2/6.68 was related to C2,6–H2,6 correlations in syringyl units. The strong signals at δC/δH 116.1/6.77 corresponded to C5–H5 correlations in the guaiacyl unit. The signal at δC/δH 130.7/7.49 was assigned to C2,6–H2,6 correlation in p-hydroxybenzoate substructures or p-coumaric acid substructures. The signal at δC/δH 40.13/2.50 may be attributed to the solvent DMSO.
In summary, the results suggested that the hemicelluloses in SCBP had a backbone of (1-4)-β-D-xylan and were mainly substituted with 4-O-methyl-D-glucuronic acid residues, and also arabinose residues and acetyl residues linked to the backbone.
SEM results
Fig. 3 shows the SEM images of the raw SCBP and hydrolysis residues. A well-defined fibre bundle with a smooth surface was observed in the raw SCBP. The raw pith differed slightly from the treated pith. This finding indicates that the structure of a cellulose unit in the solid phase remains essentially unchanged during hydrolysis. However, after hydrolysis, the SCBP was damaged to a certain extent; some materials peeled off from the surface and small holes were formed on the surface.FT-IR results
A comparative analysis of the IR spectra of SCBP before and after hydrolysis treatment is shown in Fig. 4. The characteristic peaks of raw pith at the wave numbers of 897, 1038, 1059, 1112, 1162 and 1262 cm−1 can be identified for cellulose. These peaks are consistent with the observation of previous publication.27 A sharp band developed at 897 cm−1 attributing to β-glycosidic linkages between sugar units was observed, indicating that xylan in hemicellulose of the SCBP was linked by β-form bonds. This was also in line with the observations from the 2D HSQC-NMR spectra of SCBP. The peak of the treated pith at 1262 cm−1 weakened rapidly corresponding to the discussion in the reaction mechanism that the constituent of hemicellulose in the SCBP was mostly consumed during the reaction. The peaks at 1038, 1059, 1112, 1162, 1458, and 1544 cm−1 exhibited evidently indicating that the treatment was able to remove hemicellulose so as to enhance the characteristic of cellulose and lignin contained in the hydrolysis residues. The absorbance peak at 1637 cm−1 can be identified for aldehyde or carbonyl group. This peak of hydrolysis residues wither in a significant way, meaning that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds have mostly been ruptured.
O bonds have mostly been ruptured.
Effect of hydrolysis temperature on the yield of total reducing sugars
Fig. 5 illustrates the influence of hydrolysis temperature on the reducing sugars yield. The yield of the total reducing sugars initially increased as temperature increased. These results are consistent with the observation of previous publication that higher temperature gives somewhat better yields than lower temperature.28,29 This may be attributed to the increase in the ionisation constant (kW) at a higher temperature. The concentrations of hydronium and hydroxide ions increased to break the glycosidic bonds in hemicellulose and cellulose. Once attacked by electrophilic hydrogen atoms on the glycosidic bonds, cellulose and hemicellulose can be hydrolysed in pure water. However, this reaction is very slow at ambient temperature and pressure.30 The rate of hydrolysis can be increased by increasing temperatures and pressures or by inducing acid catalysis from carbonic acid which was formed by the addition of CO2. During the acid hydrolysis of hemicellulose and cellulose, a proton is initially attached to the oxygen atom of the glycosidic bond, which slowly breaks down to give a cyclic carbeniumion. Then, free sugars, such as glucose and xylose, are liberated. The reducing sugars produced may further decompose into other products. However, as the temperature further increased, the yield of total reducing sugars decreased when the temperature exceeded 200 °C. This may have been caused by the rate of decompose of monomers becoming more rapid than the rate of hydrolysis of hemicellulose and cellulose.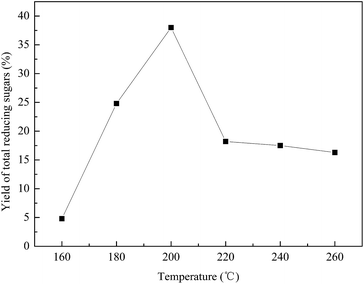 | ||
Fig. 5 Effect of hydrolysis temperature on the yield of total reducing sugars. The hydrolysis was carried out at 500 rpm for 40 min without any CO2 added. The water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCBP ratio was 30 SCBP ratio was 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
Effect of SCBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) water ratio on the yield of total reducing sugars
water ratio on the yield of total reducing sugars
The liquid/solid ratio has been recognized as an important factor in reducing sugars yield. Fig. 6 illustrates the influence of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCBP ratio on the reducing sugars yield. At first, the yield of the total reducing sugars increased as the concentration of water increased, then it reduced slightly when the amount of the SCBP decreased. These results may be attributed to the fact that at water
SCBP ratio on the reducing sugars yield. At first, the yield of the total reducing sugars increased as the concentration of water increased, then it reduced slightly when the amount of the SCBP decreased. These results may be attributed to the fact that at water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCBP ratio of 20
SCBP ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, water was too little to completely hydrolyse the hemicelluloses and cellulose. However, the ionisation constant of water increases as the concentration of water increases when water
1, water was too little to completely hydrolyse the hemicelluloses and cellulose. However, the ionisation constant of water increases as the concentration of water increases when water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCBP ratio exceeds 30
SCBP ratio exceeds 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is beneficial for the hydrolysis. As the water
1, which is beneficial for the hydrolysis. As the water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCBP ratio increased further, the concentration of the raw SCBP decreased. According to the previous report,31 as the concentrations of hemicelluloses drops, the rate of formation of oligomers slowed enough that they cannot be replenished as fast as they react to monomers. Thus, reducing sugar yield decreased at water
SCBP ratio increased further, the concentration of the raw SCBP decreased. According to the previous report,31 as the concentrations of hemicelluloses drops, the rate of formation of oligomers slowed enough that they cannot be replenished as fast as they react to monomers. Thus, reducing sugar yield decreased at water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCBP ratio above 30
SCBP ratio above 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
 | ||
Fig. 6 Effect of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCBP ratio on the yield of total reducing sugars. The hydrolysis was carried out at 200 °C and 500 rpm for 40 min without any CO2 added. SCBP ratio on the yield of total reducing sugars. The hydrolysis was carried out at 200 °C and 500 rpm for 40 min without any CO2 added. | ||
Effect of carbon dioxide on the yield of total reducing sugars
When dissolved in water, CO2 increases the availability of protons, thereby catalysing hydrolysis reactions.32 The effects of carbon dioxide on the yield of the total reducing sugars are shown in Fig. 7. A model to predict the pH of a binary CO2–H2O system in a temperature range of 100–250 °C and pressures up to a CO2 partial pressure of 150 atm (∼15.2 MPa) was proposed by Gurgel:33
pH = 8.00 × 10−6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) × T2 + 0.00209 × T − 0.216 × ln(PCO2) × T2 + 0.00209 × T − 0.216 × ln(PCO2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.92 3.92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
| (1) |
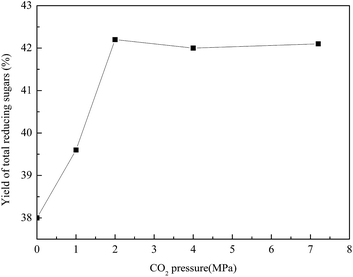 | ||
Fig. 7 Effect of CO2 on the yield of total reducing sugars. The hydrolysis was carried out at 200 °C and 500 rpm for 40 min. The water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCBP ratio was 30 SCBP ratio was 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
| CO2 partial pressure (MPa) | Reaction pressure (MPa) | pH |
|---|---|---|
| 1.0 | 2.0 | 4.0 |
| 2.0 | 4.2 | 3.9 |
| 4.0 | 7.5 | 3.7 |
| 7.5 | 12.6 | 3.6 |
Effect of stirring speed on the yield of total reducing sugars
External diffusion is reflected in the rate at which the water reaches the surface of the SCBP. The resistance effect of external diffusion focuses on the viscous flow over the pith surface. This effect can be eliminated by changing the speed of the mechanical stirrer. Fig. 8 shows the effect of stirring speed on the yield of the total reducing sugars, where the yield increased as the stirring speed increased.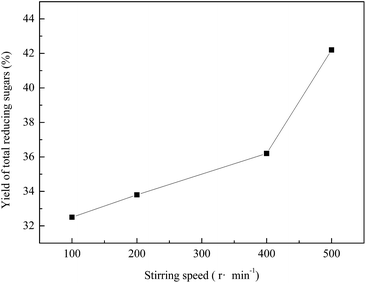 | ||
Fig. 8 Effect of stirring speed on the yield of total reducing sugars. The hydrolysis was carried out at 200 °C for 40 min with 2 MPa CO2 added. The water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCBP ratio was 30 SCBP ratio was 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
Effect of reaction time on the yield of total reducing sugars
Fig. 9 illustrates the curve of the yield of the total reducing sugars with reaction time. Initially, the yield increased as the reaction time extended. As the reaction time was further extended, the yield then decreased, as the reducing sugars produced decomposed further into other products. The highest yield of total reducing sugars was 43.6%, higher than that of reported by Prado (23.1% (ref. 35)) and Perez (15.5% (ref. 36)), in which sugarcane bagasse were treated by subcritical water. The subcritical CO2–water exhibits high capacity for dissolution and catalysis to promote the hydrolysis of SCBP. The subcritical CO2–water hydrolysis had a mild reaction condition, improved yield and was easily controlled compared with that without CO2, so that it was feasible at larger scale. | ||
Fig. 9 Effect of reaction time on the yield of total reducing sugars. The hydrolysis was carried out at 200 °C and 500 rpm with 2 MPa CO2 added. The water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SCBP ratio was 30 SCBP ratio was 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
Product distribution
Fig. 10 shows HPLC chromatograms of the product obtained at 200 °C for 50 min, with a CO2 initial pressure of 1 MPa, a SCBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio of 50
water ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and a stirring speed of 500 rpm. The HPLC peaks were identified by the comparison of sample peak retention time with the help of calibration plots for pure compounds. And the concentration profiles of the compounds in the liquid product as a function of time are shown in Fig. 11. The results showed that the main products were xylose and arabinose, the soluble hydrolysis products of hemicellulose. As hemicellulose, a higher content among the raw material SCBP, is thermally less stable than cellulose, pentose conversion was much higher than that of hexose conversion. Since xylan was the major constituent of hemicellulose, xylose was the major potential product of this process. The amount of observed glucose derived from cellulose was extremely lower. These were in agreement with the SEM and FTIR results that hydrolysis of hemi was the main process in this condition. This was corroborated by the previous publication37 that little cellulose hydrolysis was occurred below 230 °C, either in its pure form or when in lignocellulosic complexes, so that when hemicellulosic sugars recovery is intended, the process is usually carried out at 150–230 °C. Oligosaccharides such as cellobiose, cellotriose, cellotetraose and cellopentaose were observed indicating that cellulose was also partially hydrolysed. As expected, the content of oligosaccharides initially increased, then subsequently decreased as time was extended. This was because cellulose can initially be converted into water-soluble oligosaccharides and then to glucose. The contents of xylose, glucose and arabinose in the reaction medium presented the tendency of increase first and decrease afterwards. This may be attributed to the fact that xylose, glucose and arabinose generated decompose rapidly, being converted into 5-HMF, furfural and other inhibitors or unfermentable products. The high amounts of glyceraldehyde in the liquid product were likely derived from the glucuronic acid. It means that major xylan in hemicellulose was most likely O-acetyl-4-O-methylglucuronoarabinoxylan, a typical structure existing in grasses.38 Its main chain was made up of D-xylan linked by β-1,4-configuration glycosidic bond. And substituted 4-O-methyl-α-D-glucuronosyl, α-L-arabinopyranosyl, and acetyl were linked to the 1,4-linked D-xylopyranoside in the backbone located on C2 and C3 as side chains, respectively. This is also in line with the observations from 2D HSQC NMR spectra of the SCBP. The presence of acetic acid was attributed to the hydrolysis of acetyl groups linked between hemicellulosic fractions. The low amounts of ethanol in the liquid product were likely derived from the further dehydration of acetic acid. This finding was confirmed by the decrease in acetic acid concentration. The observed lactic acid was attributed to the decarboxylated of 5-HMF and furfural.
1 and a stirring speed of 500 rpm. The HPLC peaks were identified by the comparison of sample peak retention time with the help of calibration plots for pure compounds. And the concentration profiles of the compounds in the liquid product as a function of time are shown in Fig. 11. The results showed that the main products were xylose and arabinose, the soluble hydrolysis products of hemicellulose. As hemicellulose, a higher content among the raw material SCBP, is thermally less stable than cellulose, pentose conversion was much higher than that of hexose conversion. Since xylan was the major constituent of hemicellulose, xylose was the major potential product of this process. The amount of observed glucose derived from cellulose was extremely lower. These were in agreement with the SEM and FTIR results that hydrolysis of hemi was the main process in this condition. This was corroborated by the previous publication37 that little cellulose hydrolysis was occurred below 230 °C, either in its pure form or when in lignocellulosic complexes, so that when hemicellulosic sugars recovery is intended, the process is usually carried out at 150–230 °C. Oligosaccharides such as cellobiose, cellotriose, cellotetraose and cellopentaose were observed indicating that cellulose was also partially hydrolysed. As expected, the content of oligosaccharides initially increased, then subsequently decreased as time was extended. This was because cellulose can initially be converted into water-soluble oligosaccharides and then to glucose. The contents of xylose, glucose and arabinose in the reaction medium presented the tendency of increase first and decrease afterwards. This may be attributed to the fact that xylose, glucose and arabinose generated decompose rapidly, being converted into 5-HMF, furfural and other inhibitors or unfermentable products. The high amounts of glyceraldehyde in the liquid product were likely derived from the glucuronic acid. It means that major xylan in hemicellulose was most likely O-acetyl-4-O-methylglucuronoarabinoxylan, a typical structure existing in grasses.38 Its main chain was made up of D-xylan linked by β-1,4-configuration glycosidic bond. And substituted 4-O-methyl-α-D-glucuronosyl, α-L-arabinopyranosyl, and acetyl were linked to the 1,4-linked D-xylopyranoside in the backbone located on C2 and C3 as side chains, respectively. This is also in line with the observations from 2D HSQC NMR spectra of the SCBP. The presence of acetic acid was attributed to the hydrolysis of acetyl groups linked between hemicellulosic fractions. The low amounts of ethanol in the liquid product were likely derived from the further dehydration of acetic acid. This finding was confirmed by the decrease in acetic acid concentration. The observed lactic acid was attributed to the decarboxylated of 5-HMF and furfural.
Hydrolysis mechanism
Based on our studies of these products and cellulose or hemicellulose model compounds, we propose a reaction mechanism for the hydrolysis of SCBP in subcritical CO2–water, as depicted in Fig. 12. Cellulose and hemicellulose in SCBP were important bio-polymers. The reaction of subcritical CO2–water can be of interest to produce oligomers. The condition of subcritical CO2–water exhibited a high capacity for dissolution and catalysis to promote the production of oligomers. In the hydrolysis reaction of cellulose and hemicellulose, a proton was initially attached to the oxygen atom of the glycosidic bond which was then broken. As a consequence, a carbo-cation and a cellulose or hemicellulose fragment was formed. In the next step, a hydroxide ion from a dissociated water molecule attached to the carbon-cation and formed another cellulose or hemicellulose fragment, thereby producing oligosaccharides, including cellobiose, cellotriose, cellotetraose, cellopentaose, and xylobiose. Meanwhile, the group on side chain of hemicellulose was released forming arabinose, acetic acid and glucuronic acid. Glucuronic acid then rapidly decomposed and was converted into glyceraldehyde. Oligosaccharides were directly hydrolysed into pentoses and hexoses to produce predominantly xylose and glucose. Xylose, glucose and arabinose were further dehydrated to 5-HMF and furfural. 5-HMF and furfural can be decarboxylated to lactic acid.4 Conclusions
SCBP can be hydrolysed in subcritical CO2–water to produce reducing sugars. 2D HSQC NMR result suggested that the major xylan in hemicellulose of SCBP was most likely O-acetyl-4-O-methylglucuronoarabinoxylan. The SEM and FTIR results indicated that in subcritical CO2–water, hemicellulose was mainly hydrolysed with cellulose and lignin hardly being affected. A maximum reducing sugar yield of 43.6% was obtained at 200 °C, a SCBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio of 1
water ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, a CO2 pressure of up to 2 MPa, a reaction time of 50 min and a stirring speed of 500 rpm. A reaction mechanism has been proposed based on the HPLC results. Hydrolysis yielded oligosaccharides followed by further hydrolysis into xylose and glucose. Meanwhile, the group on the side chain of hemicellulose was released, forming arabinose, acetic acid, and glucuronic acid. Glucuronic acid rapidly decomposed into glyceraldehydes. The pentoses and hexoses produced were further dehydrated to 5-HMF and furfural, which were then decarboxylated into lactic acid.
30, a CO2 pressure of up to 2 MPa, a reaction time of 50 min and a stirring speed of 500 rpm. A reaction mechanism has been proposed based on the HPLC results. Hydrolysis yielded oligosaccharides followed by further hydrolysis into xylose and glucose. Meanwhile, the group on the side chain of hemicellulose was released, forming arabinose, acetic acid, and glucuronic acid. Glucuronic acid rapidly decomposed into glyceraldehydes. The pentoses and hexoses produced were further dehydrated to 5-HMF and furfural, which were then decarboxylated into lactic acid.
Acknowledgements
This work was co-financed by the National Natural Science Foundation of China (grant no. 31060102, 31560241), the Guangxi Natural Science Foundation (grant no. 2013GXNSFBA019037), the Key Laboratory of Petrochemical Resource Processing and Process Intensification Technology (grant no. 2013Z010), and Excellent Doctoral Thesis Cultivation Project of Guangxi (YCBZ2014015).Notes and references
- W. H. Chen, S. C. Ye and H. K. Sheen, Appl. Energy, 2012, 93, 237 CrossRef CAS.
- C. A. Cardona, J. A. Quintero and I. C. Paz, Bioresour. Technol., 2010, 101, 4754 CrossRef CAS PubMed.
- R. Sindhu, E. Gnansounou, P. Binod and A. Pandey, Renewable Energy, 2016, 98, 203 CrossRef CAS.
- M. M. D. S. Moretti, D. A. Bocchini-Martins, C. D. C. C. Nunes, M. A. Villena, O. M. Perrone and R. D. Silva, Appl. Energy, 2014, 122, 189 CrossRef CAS.
- R. Agrawal, R. Gaur, A. Mathur, R. Kumar, R. P. Gupta, D. K. Tuli and A. Satlewal, RSC Adv., 2015, 5, 71462 RSC.
- R. Velmurugan and K. Muthukumar, Bioresour. Technol., 2011, 102, 7119 CrossRef CAS PubMed.
- M. Adsul, B. Sharma, R. R. Singhania, J. K. Saini, A. Sharma, A. Mathur, R. Gupta and D. K. Tuli, RSC Adv., 2014, 4, 44726 RSC.
- R. Vardanega, J. M. Prado and M. A. A. Meireles, J. Supercrit. Fluids, 2015, 96, 217 CrossRef CAS.
- W. Abdelmoez, S. M. Nage, A. Bastawess, A. Ihab and H. Yoshida, J. Cleaner Prod., 2014, 70, 68 CrossRef CAS.
- J. M. Prado, T. Forster-Carneiro, M. A. Rostagno, L. A. Follegatti-Romero, F. M. Filho and M. A. A. Meireles, J. Supercrit. Fluids, 2014, 89, 89 CrossRef CAS.
- D. A. Cantero, L. Vaquerizo, F. Mato, M. D. Bermejo and M. J. Cocero, Bioresour. Technol., 2015, 179, 136 CrossRef CAS PubMed.
- J. M. Prado, D. Lachos-Perez, T. Forster-Carneiro and M. A. Rostagno, Food Bioprod. Process., 2016, 98, 95 CrossRef CAS.
- H. K. Sreenath, R. G. Koegel, A. B. Moldes, T. W. Jeffries and R. J. Straub, Process Biochem., 1999, 35, 33 CrossRef CAS.
- S. J. Moon, I. Y. Eom, J. Y. Kim, T. S. Kim, S. M. Lee and I. G. Choi, Bioresour. Technol., 2011, 102, 5912 CrossRef CAS PubMed.
- R. M. N. Roque, M. N. Baig, G. A. Leeke, S. Bowra and R. C. D. Santos, Resour., Conserv. Recycl., 2012, 59, 43 CrossRef.
- M. Goto, R. Obuchi, T. Hirose, T. Sakaki and M. Shibata, Bioresour. Technol., 2004, 93, 279 CrossRef CAS PubMed.
- Y. Zhao, W. J. Lu, H. T. Wang and J. L. Yang, Bioresour. Technol., 2009, 100, 5884 CrossRef CAS PubMed.
- M. N. Baig, R. C. D. Santos, J. King, D. Pioch and S. Bowra, Chem. Eng. Res. Des., 2013, 91, 2663 CrossRef CAS.
- G. P. V. Walsum, M. Garcia-Gil, S. F. Chen and K. Chambliss, Appl. Biochem. Biotechnol., 2007, 137–140, 301 CrossRef PubMed.
- G. P. V. Walsum and H. Shi, Bioresour. Technol., 2004, 9, 217 CrossRef PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, Determination of Structural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure (LAP), Technical Report NREL/TP-510-42618, 2012 Search PubMed.
- G. Miller, Anal. Chem., 1959, 31, 426 CrossRef CAS.
- C. S. Goh, K. T. Tan, K. T. Lee and S. Bhatia, Bioresour. Technol., 2010, 101, 4834 CrossRef CAS PubMed.
- G. L. Guo, D. C. Hsu, W. H. Chen, W. H. Chen and W. S. Hwang, Enzyme Microb. Technol., 2009, 45, 80 CrossRef CAS.
- O. Chaikumpollert, P. Methacanon and K. Suchiva, Carbohydr. Polym., 2004, 57, 191 CrossRef CAS.
- L. Moghaddam, Z. Y. Zhang, R. M. Wellard, J. P. Bartley, I. M. O'Hara and W. O. S. Doherty, Biomass Bioenergy, 2014, 70, 498 CrossRef CAS.
- Y. H. Ju, L. H. Huynh, N. S. Kasim, T. J. Guo, J. H. Wang and A. E. Fazary, Carbohydr. Polym., 2011, 83, 591 CrossRef CAS.
- W. S. L. Mok and M. J. Antal Jr, Ind. Eng. Chem. Res., 1992, 31, 1157 CrossRef CAS.
- M. Saska and E. Ozer, Biochem. Biotechnol., 1995, 45, 517 CAS.
- G. Y. Zhu, X. Zhu, Q. Fan and X. L. Wan, J. Anal. Appl. Pyrolysis, 2011, 90, 182 CrossRef CAS.
- S. E. Jacobsen and C. E. Wyman, Ind. Eng. Chem. Res., 2002, 41, 1454 CrossRef CAS.
- G. Brunner, J. Supercrit. Fluids, 2009, 47, 373 CrossRef CAS.
- L. V. A. Gurgel, M. T. B. Pimenta and A. A. D. S. Curvelo, Ind. Crops Prod., 2014, 57, 141 CrossRef CAS.
- G. Y. Zhu, X. Zhu, Q. Fan and X. L. Wan, Resour., Conserv. Recycl., 2011, 55, 409 CrossRef.
- J. M. Prado, L. A. Follegatti-Romero, T. Forster-Carneiro, M. A. Rostagno, F. M. Filho and M. A. A. Meireles, J. Supercrit. Fluids, 2014, 86, 15 CrossRef CAS.
- D. Lachos-Perez, F. Martinez-Jimenez, C. A. Rezende, G. Tompsett, M. Timko and T. Forster-Carneiro, J. Supercrit. Fluids, 2016, 108, 69 CrossRef CAS.
- S. G. Allen, L. C. Kam, A. J. Zemann and M. J. Antal, Ind. Eng. Chem. Res., 1996, 35, 2709 CrossRef CAS.
- Q. Yu, X. S. Zhuang, Z. H. Yuan, W. Wang, W. Qi and Q. Wang, J. Chem. Ind. Eng., 2012, 63, 599 CAS.
| This journal is © The Royal Society of Chemistry 2016 |