Optical thermometry of a Tm3+/Yb3+ Co-doped LiLa(MoO4)2 up-conversion phosphor with a high sensitivity
Abstract
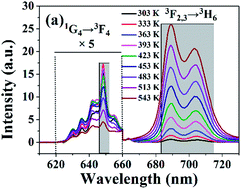
Well-crystallized LiLa(MoO4)2 co-doped with 20% Yb3+and 0.5% Tm3+ was successfully synthesized by a sol–gel method and the temperature dependence of its up-conversion (UC) luminescence was investigated systematically. The fluorescence intensity ratio between the UC emission bands centered at around 700 (Tm3+:3F2,3 → 3H6) and 650 nm (Tm3+:1G4 → 3F4) from Tm3+ was measured as a function of temperature in the range of 303–543 K under 980 nm diode laser excitation. The maximum of the relative temperature sensitivity reaches 3.85% K−1 at 300 K, which is superior to other previously reported results based on the FIR technique using the thermally coupled energy levels. This result indicates that the phosphor LiLa(MoO4)2: 0.5% Tm3+, 20% Yb3+ is a promising candidate for accurate optical temperature sensors with a higher relative sensitivity.

 Please wait while we load your content...
Please wait while we load your content...