Synthesis, structure, EPR studies and up-conversion luminescence of ZnO:Er3+–Yb3+@Gd2O3 nanostructures†
N. Babayevska*a,
B. Peplińskaa,
M. Jareka,
L. Yateb,
K. Tadyszakc,
J. Gapińskiad,
I. Iatsunskyia and
S. Jurgaa
aNanoBioMedical Centre, Adam Mickiewicz University, Umultowska 85, 61-614 Poznan, Poland. E-mail: natbab@amu.edu.pl
bCIC biomaGUNE, Paseo Miramon 182, 20009, San Sebastian, Spain
cInstitute of Molecular Physics Polish Academy of Sciences, M. Smoluchowskiego 17, 60-179 Poznań, Poland
dFaculty of Physics, Adam Mickiewicz University, Umultowska 85, 61-614, Poznań, Poland
First published on 13th September 2016
Abstract
ZnO:Er3+–Yb3+@Gd2O3 nanostructures were obtained by “wet” chemistry methods – the sol–gel technique for the preparation of ZnO and ZnO:Er3+–Yb3+ nanoparticles (NPs), and the seed deposition method for obtaining Gd2O3. The crystal structure, morphology, phase and elemental composition, resonant microwave absorption of rare earth ions, point defects in the ZnO:Er3+–Yb3+@Gd2O3 crystal structure and up-conversion luminescence were investigated by X-ray diffraction (XRD), scanning electron microscopy (SEM) with energy dispersive X-ray (EDX), X-ray photoelectron spectroscopy (XPS), electron paramagnetic resonance (EPR) spectroscopy, and optical spectroscopy. The crystallization temperature (600 °C) of the Gd2O3 phase on the ZnO surface was found. As-obtained ZnO:Er3+–Yb3+ NPs (with size ∼7 nm) are highly crystalline and monodispersed. ZnO:Er3+–Yb3+ NPs annealing at 900 °C leads to the formation of highly polydispersed ZnO:Er3+–Yb3+ NPs, covered by a Gd2O3 shell. The process of the incorporation of the rare earth ions into the ZnO structure, as well as the effect of Gd2O3 content on the morphology and visible up-conversion (UC) luminescence in ZnO:Er3+–Yb3+ matrices were studied.
1. Introduction
Zinc oxide (ZnO) is a well-known wide-band gap semiconductor, with a band gap of 3.37 eV at room temperature and a large exciton binding energy (60 meV).1,2 Un-doped and doped ZnO nanoparticles with different activators are well-known optical materials and therefore they have a wide variety of applications such as photodetectors, sensors, solar cells, light-emitting diodes and p–n heterojunctions.3–5 Furthermore, due to their good biocompatibility, low-cost, non-cytotoxicity and antibacterial properties ZnO is a very important material for medical and biological applications, mainly for food packaging, wound dressings, restorative dental materials, etc.6,7It is known that ZnO exhibits two luminescence bands: a short-wavelength band (UV region) and a broad long-wavelength band with a maximum in the green spectral range.8 Additionally, the wide band gap of ZnO allows the incorporation of luminescent centers such as rare earth (RE) ions.9 The presence of the activator and co-activator (sensitizer) in the inorganic materials (e.g. ZnO, NaGd(Y)F4) leads to formation of new donor–acceptors interactions, resulting in efficient down-conversion (DC) or up-conversion (UC) processes.10,11
Up-conversion materials are able to convert low-energy photons to higher-energy ones (UV and visible) using near infrared excitation. If luminescence of ZnO matrix depends on size and form of the materials, the emission wavelength of the UC nanoparticles is not size-dependent. Trivalent erbium ion (Er3+) in the different host matrices shows efficient UC emission in the green and orange-red spectral range. To increase the absorption cross-section and the pump efficiency, co-activation is needed. For trivalent rare earth ions such as Er3+, terbium (Tb3+) and thulium (Tm3+), the ytterbium ions (Yb3+) are an efficient sensitizer to obtaining blue, green and red emission.12,13 Thus, due to their unique electronic structure, rare earth based materials had been extensively studied, especially from the point of view of their interesting luminescence, as well as magnetic properties.
Recently, one of the most rapidly developing areas in nanotechnology is the creation and study of the hybrid core–shell heterostructures. The fabrication of the core–shell structures based on two or more types of materials as well as the variation of their properties allows developing new multifunctional platforms for wide scientific applications.14,15
To obtain effective functional materials (phosphor, collar cells, biomarker, etc.) it is needed to control the materials characteristics, such as crystal structure, phase and chemical composition, particles morphology, type and concentration of the activator. “Wet” chemistry methods (sol–gel, co-precipitation, hydrothermal, microemulsion, etc.) are well-known methods, which make possible to obtain highly crystalline nanoparticles below 10 nm with controlled shape, size and dispersity.16,17 Moreover, an increasing number of publications are devoted to the synthesis and characterization of the micro- or nanomaterials, doped with luminescent ions or dopants, which modify the crystal structures.18,19 However, the mechanisms of the activator incorporation into the crystal lattice are not fully understood and the aqueous synthesis of the nanoparticles with strictly stoichiometry, especially when the synthesis occurs in the multi-component system, needs the strict control of the synthesis process. Non-controlled synthesis can lead to formation of undesired phases or groups, adversely affecting the functional properties of the final material. Theoretical ions concentration of precursors and real ions concentration in final materials may be different, because part of the activator/dopant ions are incorporated in the crystal structure), as well as part of the ions remains in solution (supernatant) or in the “dead volume” (walls and bottom of the laboratory glassware). Therefore, the incorporation coefficients (segregation coefficient in case of the single crystals) of the activator/dopant for each material is different20,21 and it is very important to know the real ion concentration in the final material.
At the same time, it should be noted, that the surface of the nanoparticles, including ZnO, is full of defects, so adsorption of OH− or CO32− groups (from surrounding synthesis environment) is evident. To prevent absorption processes the surface of nanocrystals needs to be modified by a protective shell based on metal oxide, e.g. Gd2O3.22
Obtained ZnO:Er3+–Yb3+@Gd2O3 nanoparticles, due to their up-conversion properties, as well as biocompatibility and non-toxicity of the ZnO matrix, can find wide applications in biomedicine. They can be good alternative to well-known organic biomarkers, based on lipids or semiconductors quantum dots (QDs), which consist of toxic metals. Unlike UV excitation, which is harmful for living organisms, up-conversion nanoparticles produces near-infrared (NIR) light-excited fluorescence which is not toxic for in vivo studies. Also, due to the paramagnetic properties of the gadolinium ions, they can be used as contrast agents, useful for magnetic resonance imaging, MRI. Furthermore, electron paramagnetic resonance spectroscopy (EPR) will allow to detect rare earth ions and paramagnetic structural defects in the crystal structure. The presence of structural defects can be an indicator of the synthesis quality of ZnO structures, which could be an important factor when applied in vivo experiments.
In this paper we report the sol–gel synthesis of the nanostructures, based on ZnO nanoparticles, doped with Er3+ and Yb3+ up-conversion ions and covered by Gd2O3 shell. The process of the incorporation of rare earths ions into the ZnO structure, as well as the effect of Gd2O3 content on the crystalline structure, morphology, visible up-conversion (UC) luminescence in the ZnO:Er3+–Yb3+ matrices are studied.
2. Results and discussion
2.1. Structural characteristics
| Samples title | Samples preparation |
|---|---|
| S-I | As-obtained ZnO NPs (synthesis temperature 60 °C) |
| S-II | ZnO NPs annealed at 900 °C for 1 h |
| S-III | ZnO:Er3+–Yb3+ NPs annealed at 900 °C for 1 h |
| S-IV | ZnO:Er3+–Yb3+ NPs modified by Gd3+ shell for 6 h and annealed at 900 °C for 1 h (ZnO:Er3+–Yb3+@Gd2O3 core–shell) |
| S-V | ZnO:Er3+–Yb3+ NPs modified by Gd3+ shell for 48 h and annealed at 900 °C for 1 h (ZnO:Er3+–Yb3+@Gd2O3 core–shell) |
| S-VI | ZnO:Er3+–Yb3+ NPs modified by Gd3+ shell for 96 h and annealed at 900 °C for 1 h (ZnO:Er3+–Yb3+@Gd2O3 core–shell) |
The as-obtained ZnO NPs (S-I) obtained by sol–gel technique have spherical shape, are high crystalline and monodispersed with the mean size around 7 nm. Doping ZnO matrix with Er3+ and Yb3+ does not change the form and size of the nominal pure ZnO NPs (Fig. S1, ESI†). On the other hand, the annealing of the ZnO, ZnO:Er3+–Yb3+, and ZnO:Er3+–Yb3+@Gd2O3 samples at 900 °C for 1 h leads to a nanoparticle sintering and to a strong particle growth due to recrystallization process.
Fig. 1 presents the SEM images of the ZnO NPs doped with Er3+ and Yb3+ and ZnO@Gd2O3 which show nanostructured morphologies. After the annealing, the ZnO:Er3+–Yb3+ (S-III) particles are polydispersed with sizes around 30-300 nm. Surface modification of ZnO:Er3+–Yb3+ NPs by Gd(NO3)3 water solution (for 96 h) and following annealing at 900 °C for 1 h leads to blocking of the particles growth and formation of monodispersed nanoparticles with sizes around 50 nm (S-IV–VI). Thus, we can conclude, that the morphology of the obtained annealed ZnO:Er3+–Yb3+@Gd2O3 core–shell nanostructures is not depended from Gd2O3 content.
 | ||
| Fig. 2 The phase composition of ZnO@Gd2O3 nanostructures, annealed at different temperatures: a – 500 °C, b – 600 °C, c – 900 °C for 1 h, inset – range at 27–30 2θ degrees at a higher magnification. | ||
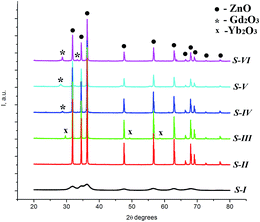
The diffraction peaks in whole temperature range, correspond to the main (100), (002), (101), (102), (110), (103), (112), (201), and weak (200), (004) and (202) crystal planes, belonging to hexagonal wurtzite ZnO structure with P63mc space group (corresponding to the standard crystallographic data in the JCPDS-ICDD index card no. 36-1451). For all samples the most intense peak was the one attributed to the (101) planes of the wurtzite structure. At 500 °C no additional phases were found. Annealing of the sample at 600 °C (Fig. 2b and more detailed in inset) leads to the appearing of the peak with low intensity attributed to the (222) planes, corresponding to the beginning of the cubic Gd2O3 phase crystallization. At 900 °C the peak attributed to the (222) planes is more intense and a new small peak corresponding to the (400) plane of the Gd2O3 phase is observed. Thus, for further experiments 900 °C was chosen as the optimal temperature.
Fig. 3 presents the XRD patterns of the ZnO NPs (as-prepared), as well as ZnO NPs, ZnO:Er3+–Yb3+ NPs, and ZnO:Er3+–Yb3+@Gd2O3 core–shell nanostructures, annealed at 900 °C. The broad peaks for the sample (S-I) are due to the small particle size of the as-obtained ZnO NPs.23 Diffraction peaks for all annealed samples (where the NPs size increases up to 50 nm, samples S-II–S-VI) are intense and narrow indicating that these particles are highly crystalline. Doping ZnO matrix with Er3+ and Yb3+ ions leads to a small shift (within the measurement error) of the diffraction peaks to lower angles. For the ZnO:Er3+–Yb3+ sample (S-III) some small peaks attributed to Yb2O3 are found. But after the Gd2O3 shell modification (S-IV–VI), the diffraction pattern consists of only the ZnO and Gd2O3 crystalline structures. The Gd2O3 cubic phase was observed after just 6 h of ZnO NPs surface modification by Gd3+ and annealing (S-IV). The peak positions for all samples did not change, indicating that Gd3+ ions are not being incorporated into the ZnO structure, in other words, the Gd2O3 shell covered the ZnO NPs surface with formation of the two independent ZnO and Gd2O3 crystalline phases.
2.2. Elemental composition
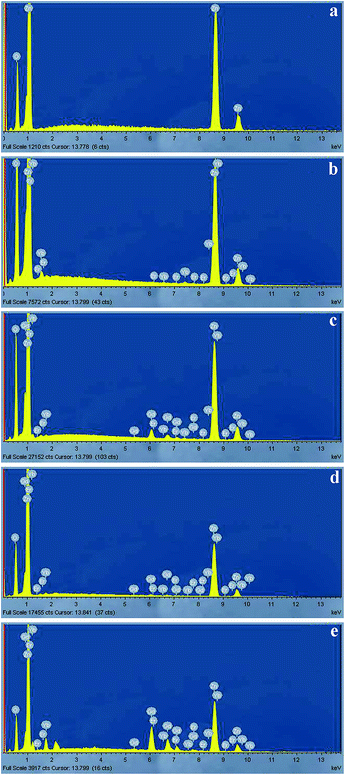
The EDX results show, that the incorporation coefficients for both rare earth ions (Er3+ and Yb3+) are very low and reach approximately 1%. This is due to the fact, that in “wet” synthesis after centrifugation and washing, part of the RE ions is incorporated into the crystal, but part of the RE ions remains in the solution (supernatant) or in “dead volume” (walls and bottom of the laboratory glassware). Depending on the modification time of the ZnO:Er3+–Yb3+ NPs in Gd(NO3)3 water solution and subsequent annealing, Gd as well as O contents are increased due to formation of the corresponding oxides.
Elemental mapping of Zn, Gd and O, shows homogeneous distribution of all components without any concentrated places at the surface (Fig. S2, ESI†). The resolution of the SEM mapping is not able to detect the presence of the Gd2O3 shell. By analogy with the previously obtained results, related to un-doped ZnO@Gd2O3 nanostructures,23 we can conclude that shell formation of doped ZnO:Er3+–Yb3+@Gd2O3 nanostructures is similar and the Gd2O3 shell thickness is approximately of 3 nm (Fig. S3, ESI†). Moreover, the elemental analysis in the core and shell region indicates, that amount of the Gd is higher in the shell region (Fig. S4 and S5 ESI†).
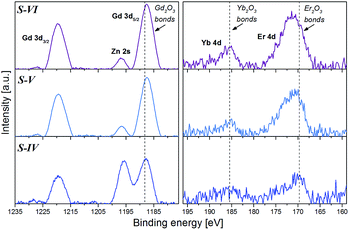 | ||
| Fig. 5 XPS Gd 3d, Yb 4d and Er 4d spectra of the ZnO:Er3+–Yb3+ NPs modified by Gd3+ shell for 6 h (S-IV), 48 h (S-V) and 96 h (S-VI) and annealed at 900 °C for 1 h (ZnO:Er3+–Yb3+@Gd2O3 core–shell). | ||
The Yb 4d and Er 4d peak positions are around 185.4 and 170.5 eV, respectively, which can be correlated to Yb–O and Er–O bonds in Yb2O3 and Er2O3 (ref. 26) materials, and are in agreement with the expected presence of Er3+ and Yb3+ ions. The Zn 2p signal from the zinc in the core structure was also recorded and showed in Fig. S6 (ESI†). The Zn 2p3/2 peak position in the samples is located at around 1021.8 eV and can be correlated to Zn–O bonds in ZnO.26 Thus, the XPS results are in agreement with the XRD data and confirm the presence of ZnO, Er3+, Yb3+ and Gd2O3 in the ZnO:Er3+–Yb3+@Gd2O3 core–shell samples. The relative chemical compositions of the S-IV to S-VI samples, listed in Table 2, obtained from the areas of the photoelectron peaks reveal that the Er and Yb elements are in a low proportion, up to 0.8 at% for the Er and 0.2 at% for the Yb. There is an increase in the Gd, Er and Yb relative compositions from the sample S-IV to the S-VI, which correlates well with the modification of the samples with the Gd3+ at different times.
| Sample | Gd (at%) | Zn (at%) | O (at%) | C (at%) | Er (at%) | Yb (at%) |
|---|---|---|---|---|---|---|
| S-IV | 2.4 | 47.4 | 41.7 | 8.2 | 0.2 | 0.1 |
| S-V | 10.3 | 27.0 | 47.5 | 14.4 | 0.7 | 0.2 |
| S-VI | 10.9 | 28.2 | 45.8 | 14.2 | 0.8 | 0.2 |
2.3. Electron paramagnetic resonance spectroscopy (EPR) analysis
Obtained ZnO:Er3+–Yb3+ NPs and ZnO:Er3+–Yb3+@Gd2O3 core–shell nanoparticles were studied by EPR. The system gives rise to multiple EPR signals of various sources, namely, Gd3+ ions (electron conf. [Xe] 4f75s2p6, ground term 8S7/2, electron spin S = 7/2) in the shell material (Gd2O3), Er3+ ions (electron conf. [Xe]4f115s2p6, ground term 4I15/2, S = 3/2) and Yb3+ ions (electron conf. [Xe]4f135s2p6, ground term 2F7/2, S = 1/2) doped in ZnO, as well as ZnO defects.The most dominant contribution in the EPR spectrum is coming from gadolinium oxide (Gd2O3).27 The shell exhibits very broad EPR line of ∼260 mT at 295 K and gets broader to ∼300 mT at 4.2 K (S-IV) and 335 mT (S-VI), with effective g-factor ∼2.0. The dipol–dipol interactions between Gd3+ ions even are strong enough to broaden the line to the extent that the internal line structure is indistinguishable (Fig. 6). In this case, the situation is similar to that in copper(II) sulfate pentahydrate but due to the higher spin the interactions are stronger and leading to bigger line broadening than in the case of copper. Even, though 32 out of 80 atoms in the unit cell are Gd3+ ions, the interactions leading to collective magnetism were not observed. Field cooling and zero field cooling experiments showed no bifurcations until 2 K.28 The small interactions between paramagnetic ions were simulated with a Curie–Weiss curve with small value of the Θ parameter.28
 | ||
| Fig. 6 Normalized EPR spectra of Gd2O3 (black line) and ZnO (red line) powders recorded with polydopamine standard (5.5 × 1015 spins g = 2.0036, T = 295 K). | ||
For comparison as EPR standard polydopamine radicals were used. The relative signal strength can be compared between ZnO defects and Gd2O3 (Fig. 6).
The X-band EPR spectrum usually consists of three main lines at ge ≈ 6.0, ge ≈ 2.8 and ge ≈ 2.29 The increasing amount of Gd3+ ions in the structure strongly broadens the lines, which end up as one broad line without resolved fine structure. It is showed that 1.95 × 1021 ions per cm3 is enough to broaden the lines at ge ≈ 2.8 and ge ≈ 2. In our case we do not observe even the last vanishing transition at ge ≈ 6.0.29
ZnO is visible in EPR due to the defect centres appearing in the structure during the synthesis of the nanocrystals. Possible defect centres in ZnO are: zinc vacancies, zinc on interstitial sites (visible Zn+), oxygen on interstitial sites (O−), and oxygen vacancies (visible: Vo2+).30 The EPR linewidth of ZnO is 79 mT with effective g-factor of 2.1 (N = 6.3 × 1018 spins) (Fig. 6). The origin of the ZnO signal is related with thermally stable defects.30
Fig. 7 depicts the transitions in the ZnO:Er3+–Yb3+ system, especially in the Er3+and Yb3+ spin system. It can be seen that the line structure is better resolved in liquid helium temperatures. The rare earth ions usually present fast relaxation times causing line broadening and are not resolved well at room temperatures. For example the EPR signal from Yb3+ ion is visible in liquid helium temperatures in systems like KZnF3 where the Yb3+ ion substitutes Zn2+.31 The Er3+ ion was observed in liquid helium temperatures, 5.2 K, in SrTiO3 single crystals32 and YAl3(BO3)4 and EuAl3(BO3)4 until 80 K.33 It is assumed that the strong increase of the EPR signal intensity in liquid helium temperatures in comparison to the signal observed in room temperature is due to the lanthanides doping in the ZnO structure. The large mismatch and line broadening of lines can be the result of dipolar interaction between ions, different mixed atom coordination, and multiple phases like Er2O3 in the ZnO system.
2.4. Photoluminescence study
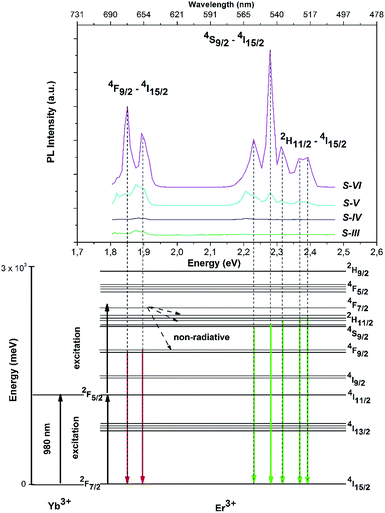
Fig. 8 shows the UC photoluminescence (PL) spectra of the ZnO:Er3+–Yb3+@Gd2O3 core–shell nanostructures. It is clearly seen that upon excitation by 980 nm S-III (ZnO:Er3+–Yb3+) and S-IV (ZnO:Er3+–Yb3+ modified with Gd2O3 for 6 h) revealed very weak up-conversion in the range of 515–570 nm and 630–685 nm. The increasing of the surface modification time (increasing Gd2O3 content), S-V and S-VI samples, leads to the enhance of the up-conversion emission. Pokhrel et al. have shown that these PL peaks correspond to radiative transitions from 4F9/2 to 4I15/2 (red emission) and from 4S9/2, 2H11/2 to 4I15/2 (green emission) of Er3+ ion.34 The splitting structure of PL peaks might be explained by the Stark effect (the splitting and shifting of energy levels due to presence of an electric field) of Er3+ energy levels induced by the crystal field of Gd2O3.22It is possible to describe the mechanism of the excitation and emission using the energy diagram (Fig. 8, bottom image). It is well-known that Yb3+ has a larger absorption cross-section at 980 nm than Er3+.35 Thus, Er3+ can resonantly receive energy from Yb3+, so the transition of 4I15/2–4I11/2 and 4I11/2–4F7/2 are excited and the UC emission is significantly enhanced.36 Afterward, non-radiative transitions to 2H11/2 and 4S3/2 produce green emission and to 4F9/2 produce red emission.
There are two possible mechanisms to explain the increasing of UC emission due to the surface modification (the increase of the Gd2O3 content). Firstly, the surface modification annihilates the dangling bonds and attaching groups at the surface of nanoparticles which can quench the PL emission. Secondly, core/shell structure increases the distance between luminescent Er3+ ions and surface defects, thus blocking the non-radiative pathway.
We can also notice that the red emission prevails for the S-V sample, while in sample S-VI the red emission is suppressed and the green PL is enhanced. The same effects and their luminescence mechanisms were described by X. Meng and co-workers.22 Using the theory of multi-phonon relaxation rate, Meng et al. have shown that emission through 4S9/2, 2H11/2 transition is more influenced by the Yb3+ concentration. Therefore, the increasing of the Gd2O3 content leads to dominating of green PL.
3. Experimental
3.1. Synthesis of the ZnO:Er3+–Yb3+@Gd2O3 core–shell nanostructures
Gd2O3 shell was prepared by seed deposition method.36 Part of ZnO:Er3+–Yb3+ NPs were immersed into 0.1 mol l−1 Gd(NO3)3 solution for a period of 6 h to 96 h. Obtained core–shell nanostructures were separated, washed with deionized water (18 MΩ cm) and dried in an excicator. To obtain gadolinium oxide phase (Gd2O3), nanoparticles were annealed at 900 °C in air for 1 h.
3.2. Characterization
The morphology and chemical composition of the samples were studied by scanning electron microscopy (SEM, JEOL, JSM-7001F) equipped with an energy dispersive X-ray (EDX) analyzer. Powder X-ray diffraction (XRD) studies of the powder samples were carried out on an Empyrean (PANalytical) diffractometer using Cu Kα radiation (λ = 1.54 Å), reflection-transmission spinner (sample stage) and PIXcel 3D detector, operating in the Bragg–Brentano geometry. The 2Theta scans were recorded at room temperature in angles ranging from 20 to 80 (°2Theta) with a step size of 0.006 (°2Th.) and continuous scan mode. XPS experiments were performed in a SPECS Sage HR 100 spectrometer with a non-monochromatic X-ray source (Aluminum Kα line of 1486.6 eV energy and 350 W). The samples were placed perpendicular to the analyzer axis and calibrated using the 3d5/2 line of Ag with a full width at half maximum (FWHM) of 1.1 eV. An electron flood gun was used to compensate for charging during XPS data acquisition. The selected resolution for the spectra was 10 eV of pass energy and 0.15 eV per step for the high resolution spectra. All measurements were made in an ultra-high vacuum (UHV) chamber at a pressure below 8 × 10−8 mbar. The spectroscopic EPR measurements were performed with a RADIOPAN SX spectrometer equipped with an Oxford CF935 cryostat allowing measurements in the temperature range 4.2–300 K. The modulation amplitude was set to 0.1 mT, the microwave power was 11.38 mW. The visible UC emission was excited at 980 nm and at room temperature by means of the LSM 780 NLO confocal system (Zeiss) working in the lambda mode. Infrared light was delivered by tunable femtosecond Chameleon laser (Coherent) coupled to the LSM 780 system. Spectral analysis was performed on the light emitted by representative grains of the powder samples.4. Conclusions
In summary, by using the sol–gel and seed deposition techniques ZnO@Gd2O3 and ZnO:Er3+–Yb3+@Gd2O3 core–shell nanostructures were obtained. The size of nanoparticles increased from 7 nm (individual as-obtained ZnO:Er3+–Yb3+ NPs) to 50 nm after Gd2O3 shell formation (core–shell nanostructures). XRD data showed, that Gd2O3 phase began to crystallize at 600 °C. In the ZnO:Er3+–Yb3+ NPs a secondary phase of Yb2O3 was found. Real RE concentrations in ZnO:Er3+–Yb3+@Gd2O3 crystal lattice were determined. EPR investigation allowed estimating the presence of three different signals which are listed here in descending order of appearance: gadolinium oxide Gd2O3, ZnO structure defects, and at liquid helium temperatures also Er3+,Yb3+ ions. The presence of the Gd2O3 protective shell on the ZnO:Er3+–Yb3+ surfaces led to an increase of the up-conversion luminescense intensity of the ZnO matrix due to a decreasing of the structural defects. The obtained ZnO@Gd2O3 and ZnO:Er3+–Yb3+@Gd2O3 core–shell nanostructures with effective green and red luminescence will have potential application as efficient phosphors for wide scientific and medical applications.Acknowledgements
Financial support from the National Centre for Research and Development under research grant “Nanomaterials and their application to biomedicine”, contract number PBS1/A9/13/2012, is gratefully acknowledged.References
- R. Viter, A. A. Chaaya, I. Iatsunskyi, G. Nowaczyk, K. Kovalevskis, D. Erts, P. Miele, V. Smyntyna and M. Bechelany, Nanotechnology, 2015, 26, 105501 CrossRef PubMed.
- E. Robak, E. Coy, M. Kotkowiak, S. Jurga, K. Załęski and H. Drozdowski, Nanotechnology, 2016, 27, 175706 CrossRef PubMed.
- N. Plank, M. Welland, J. MacManus-Driscoll and L. Schmidt-Mende, Thin Solid Films, 2008, 516, 7218 CrossRef CAS.
- M. Vafaee, M. Ghamsari and S. Radiman, J. Lumin., 2011, 131, 155 CrossRef CAS.
- A. Stafiniak, B. Boratyński, A. Baranowska-Korczyc, A. Szyszka, M. Ramiączek-Krasowska, J. Prażmowska, K. Fronc, D. Elbaum, R. Paszkiewicz and M. Tłaczała, Sens. Actuators, B, 2011, 160, 1413 CrossRef CAS.
- M. Arefi, S. Rezaei-Zarchi and S. Imani, Afr. J. Biotechnol., 2012, 11(34), 8520 CAS.
- S. Divyapriya, C. Sowmia and S. Sasikala, World J. Pharm. Res., 2014, 3, 1635 Search PubMed.
- G. Kenanakisa, M. Androulidaki, E. Koudoumas, C. Savvakis and N. Katsarakis, Superlattices Microstruct., 2007, 42, 473 CrossRef.
- E. Wolska, J. Kaszewski, P. Kiełbik, J. Grzyb, M. Godlewski and M. Godlewski, Opt. Mater., 2014, 36, 1655 CrossRef CAS.
- A. Chelouche, T. Touam, D. Djouadi and A. Aksas, Optik, 2014, 125, 5626 CrossRef CAS.
- R. John and R. Rajakumari, Nano–Micro Lett., 2012, 4(2), 65 CrossRef CAS.
- W. Wang and K. Chen, J. Phys. Chem. C, 2013, 117, 23716 Search PubMed.
- F. Wang, R. Deng and X. Liu, Nat. Protoc., 2014, 9(7), 1634 CrossRef CAS PubMed.
- M. Ismail, W. Cao and M. Humadi, Optik, 2016, 127, 4307 CrossRef CAS.
- R. Viter, I. Iatsunskyi, V. Fedorenko, S. Tumenas, Z. Balevicius, A. Ramanavicius, S. Balme, M. Kempiński, G. Nowaczyk, S. Jurga and M. Bechelany, J. Phys. Chem. C, 2016, 120, 5124–5132 CAS.
- W. Beek, M. Wienk, M. Kemerink, X. Yang and R. Janssen, J. Phys. Chem. B, 2005, 109(19), 9505 CrossRef CAS PubMed.
- Y. Shi, C. Zhu, L. Wang, C. Zhao, W. Li, K. Fung, T. Ma, A. Hagfeldt and N. Wang, Chem. Mater., 2013, 25, 1000 CrossRef CAS.
- N. Babayevskaya, Y. Savvin and A. Tolmachev, Inorg. Mater., 2007, 43(8), 873 CrossRef CAS.
- N. Babayevskaya, Y. Savin and A. Tolmachev, Funct. Mater., 2006, 13(1), 90 CAS.
- A. Kosinova, M. I. Kolybaeva, O. N. Bezkrovnaya, V. F. Tkachenko, E. V. Grishina, A. N. Levchenko, V. M. Puzikov and I. M. Pritula, Cryst. Res. Technol., 2014, 49, 965 CrossRef CAS.
- O. Sidletskiy, A. Belsky, A. Gektin, S. Neicheva, D. Kurtsev, V. Kononets, C. Dujardin, K. Lebbou, O. Zelenskaya, V. Tarasov, K. Belikov and B. Grinyov, Cryst. Growth Des., 2012, 12, 4411 CAS.
- X. Meng, C. Liu, F. Wu and J. Li, J. Colloid Interface Sci., 2011, 358, 334 CrossRef CAS PubMed.
- N. Babayevska, G. Nowaczyk, M. Jarek, K. Załęski and S. Jurga, J. Alloys Compd., 2016, 672, 350 CrossRef CAS.
- S. Cotton, Lanthanide and actinide chemistry, Wiley, 2006 Search PubMed.
- F. Söderlind, H. Pedersen, R. Petoral Jr, P. Käll and K. Uvdal, J. Colloid Interface Sci., 2005, 288, 140 CrossRef PubMed.
- J. Moulder, W. Stickle, P. Sobol and K. Bomben, Handbook of x-ray photoelectron spectroscopy: a reference book of standard spectra for identification and interpretation of XPS data, Physical Electronics, 1995 Search PubMed.
- S. Misra, M. Bartkowski and P. Mikolajczak, J. Chem. Phys., 1983, 78, 5369 CrossRef CAS.
- P. Pascuta, M. Bosca, M. Culea, S. Simon and E. Culea, Mod. Phys. Lett. B, 2008, 22, 447 CrossRef CAS.
- C. Azzoni, D. Martino, A. Paleari, A. Speghini and M. Bettinelli, J. Mater. Sci., 1999, 34(16), 3931 CrossRef CAS.
- H. Kaftelen, K. Ocakoglu, R. Thomann, S. Tu, S. Weber and E. Erdem, Phys. Rev., 2012, 86, 014113 CrossRef.
- M. Falin, K. Gerasimov, V. Latypov, A. Leushin, S. Schweizer and J.-M. Spaeth, J. Phys. Chem. Solids, 2015, 77, 157 CrossRef CAS.
- A. Skvortsov, D. Savchenko, Z. Potucek, L. Jastrabik, V. Trepakov and A. Dejneka, Phys. Status Solidi B, 2014, 251, 2270 CrossRef CAS.
- A. Prokhorov, A. Prokhorov, L. Chernysh, P. Aleshkevich, V. Dyakonov and H. Szymczak, J. Magn. Magn. Mater., 2013, 326, 162 CrossRef CAS.
- M. Pokhrel, G. A. Kumar and D. K. Sardar, J. Mater. Chem. A, 2013, 1, 11595 CAS.
- C. Strohhöfer and A. Polman, J. Appl. Phys., 2001, 90, 4314 CrossRef.
- C. C. Han, Y. Du, X. Meng, F. Wu and Y. Fang, Appl. Surf. Sci., 2013, 274, 60 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18393j |
| This journal is © The Royal Society of Chemistry 2016 |