Umbelliferone attenuates lipopolysaccharide-induced acute lung injury linked with regulation of TLRs–MyD88 and RIP140/NF-κB signaling pathways†
Fen Luo†
a,
Rui Zhou†a,
Hui Leib,
Yi Mouc,
Ping Zhangd,
Yi Sunf,
Tong Chen ef,
Ling He*f and
Tianhua Yan*a
ef,
Ling He*f and
Tianhua Yan*a
aDepartment of Physiology and Pharmacology, China Pharmaceutical University, Nanjing 210009, China. E-mail: yantianhuabest@126.com
bPharmaceutical Experiment Center of China Pharmaceutical University, Nanjing 211198, China
cTaizhou University, Taizhou 225300, China
dDepartment of Pharmaceutics, China Pharmaceutical University, Nanjing 210009, China
eState Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing 210009, China
fDepartment of Pharmacology, China Pharmaceutical University, Nanjing 210009, China. E-mail: heling92@hotmail.com
First published on 21st September 2016
Abstract
Umbelliferone (Umb), isolated from the chloroform fraction of Potentilla evestita, exerts a variety of pharmacological activities. The aim of the present study was to evaluate the protective effects and possible mechanisms of Umb on lipopolysaccharide (LPS)-induced acute lung injury (ALI) in mice. Male BALB/c mice were randomly divided into five groups: control group, LPS group, LPS + dexamethasone (Dex, 2 mg kg−1) group, LPS + Umb (20 mg kg−1) group, and LPS + Umb (Umb, 40 mg kg−1) group. Umb and Dex were orally administered 15 min before the intra-tracheal (IT) administration of LPS. 6 h later, the mice were sacrificed. The lung tissues and bronchoalveolar fluid (BALF) were prepared for further analysis. Our results showed that pretreatment with Umb prior to the LPS challenge significantly decreased the lung W/D weight ratio, total leukocyte number and neutrophil percentage in the BALF. Umb also reduced pulmonary MPO activity and alleviated histopathological alteration. Besides, the contents of inflammatory cytokines including interleukin (IL)-6, interleukin (IL)-1β and tumor neurosis factor (TNF)-α were also found to be significantly inhibited by Umb in BALF. Furthermore, the expressions of TLR2, TLR4, MyD88, receptor-interacting protein 140 (RIP140), RelA, CBP, nuclear factor kappa B (NF-κB), caspase-9, caspase-3, Bax in lung tissues were inhibited and Bcl-2 expression was increased in the LPS + Umb group. These results showed that administration of Umb could attenuate LPS-induced ALI, possibly via the anti-inflammatory and anti-apoptotic activities through the TLRs–MyD88 and RIP140/NF-κB pathway.
1. Introduction
Acute lung injury (ALI), or its more severe form acute respiratory distress syndrome (ARDS), is characterized by severe hypoxemia, pulmonary edema and neutrophil accumulation in the lungs. ALI is a common clinical problem associated with significant morbidity and mortality.1 Although ALI is a major clinical problem with a high mortality rate of 30% to 40%, and various advances in antimicrobial therapy have been made in the past few decades, there are still limited effective measures or specific medicines to treat ALI.2Inflammatory cytokines are the major mediators in the pulmonary disorders including ALI.3 These acute inflammatory responses can be summarized as leakage of protein into the alveolar space, inflammatory cells accumulation, interstitial edema, and disruption of epithelial integrity.4 Nuclear receptor-interacting protein 1 (NRIP1), also known as receptor-interacting protein 140 (RIP140), is widely expressed and participates in several physiological responses.5 RIP140 is originally identified as a modulator of oestrogen receptor activity in breast cancer cells and has been recently found to promote and regulate inflammatory processes by activating the expression of pro-inflammatory cytokines.6 RIP140 interacts with the nuclear factor-kappa B (NF-κB) subunit RelA or cAMP-response element binding protein (CREB)-binding protein (CBP) to induce the production of the cytokines.7 The NF-κB activation caused by bacterial endotoxin is required for the transcription and generation of pro-inflammatory mediators including tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 in the early phase of acute lung injury.8 Additionally, dysregulation of apoptotic pathways is thought to play an important role in the pathogenesis of ALI for the loss of normal functional cells in lungs.9 Former literature proposed that the epithelial/endothelial cell apoptosis contributed to the endothelial and epithelial injury which was the key feature of ALI in humans.10 Accumulating evidence indicated that apoptosis inhibitors increased survival rate of rodents in ALI models.11
As an critical component of outer membrane in bacterial, LPS induces acute lung injury marked by the overproduction of numerous pro-inflammatory cytokines.12 The intratracheal administration of lipopolysaccharide (LPS) had been extensively used to study the pathogenesis and intervention of ALI in mice.13 Recently, the interventions aimed at modulating TLR4–NF-κB signaling and the alleviating apoptosis process may have potential therapeutic advantage for ALI.14
Potentilla evestita belongs to the family Rosaceae and is distributed in the eastern Himalayan range from Indus to Kumaon, in Arctic, Alpine and temperate regions of the Northern hemisphere. In Pakistan, it is usually found in Gilgit regions. Several medicinal uses of P. evestita have been reported, such as analgesic, anti-microbial, anti-inflammatory, anti-diabetic, anti-cancer, anti-spasmodic and hepatoprotective effects. Umbelliferone (Umb), isolated from the chloroform fraction of Potentilla evestita, is a widely distributed natural product in carrot, coriander, garden angelica and edible fruits of golden apple (Aegle marmelos Correa).15 Umb exhibited various properties including hepatoprotective,16 anti-ischemia,17 anti-depressive,17 anti-diabetic,18 anti-cancer,19 anti-oxidative and anti-inflammatory activities.20 It is noteworthy that Umb could attenuate allergic airway inflammation in the previous research.21 However, there has been limited literature to elucidate the pharmacological efficacy of Umb on acute lung injury to date. The present study was aimed to investigate the protective effect of Umb on LPS-induced ALI and explore its possible mechanisms through anti-inflammation and anti-apoptosis in vivo.
2. Methods and materials
2.1. Reagents
Umbelliferone (7-hydroxycoumarin, purity: 99%) was purchased from National Institutes for Food and Drug Control (Beijing, China). Dexamethasone (Dex) was obtained from Xiansheng drug Store (Nanjing, China). All the drugs were dissolved in DMSO and the final concentration of DMSO was less than 0.1% [v/v] in all the experiment. LPS was produced by Sigma-Aldrich (St. Louis, MO, USA). Myeloperoxidase (MPO) and Wright–Giemsa staining commercial kits were purchased from the Institute of Jiancheng Bioengineering (Nanjing, China). The enzyme-linked immunosorbent assay (ELISA) kits for determination of IL-6, IL-1β and TNF-α were produced by Nanjing KeyGEN Biotech. Co., Ltd (Nanjing, China). Antibodies of caspase-3 (#9665), caspase-9 (#9508), NF-κB (#4882), Bax (#5023), Bcl-2 (#2870), TLR4 (#2246), TLR2 (#2229) and MyD88 (#3699) were purchased from Cell Signaling Technology (Danvers, USA). RIP140 (#sc-8997), RelA (#sc-81622) and CBP (#sc-25748) were supplied from Santa Cruz Biotechnology.2.2 Animals
Male BALB/c mice (18–22 g) were obtained from Jiangning Qinglongshan Animal Cultivation Farm (Nanjing, China). Mice were maintained in an animal facility under standard laboratory conditions for 1 week prior to experiments. The animals were provided with water and standard chow ad libitum. All experimental procedures were carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The present experiments were also approved by China Pharmaceutical University (no. CPU-TCM-2013012) Medicine Animal Care and Use Committee.2.3 Pharmacokinetic analysis of Umb in LPS-induced lung injury mice
24 mice had free access to water and rodent chow 12 h before drug administration. The animals were randomly divided into eight time point groups: 5 min group, 10 min group, 15 min group, 30 min group, 60 min group, 120 min group, 240 min group and 360 min group. The mice were intragastrically administered with 40 mg kg−1 Umb. 5, 10, 15, 30, 60, 120, 240 or 360 min later, the mice were anesthetized with chloraldurate (3%), and then intratracheally administrated 20 μg of LPS dissolved in 50 μl phosphate buffered saline (PBS) to induce ALI. 6 h after each time point, three mice were sacrificed, lung tissues were collected and stored at −80 °C for further UPLC analysis.50 mg lung tissues were homogenized, dissolved with acetonitrile at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and centrifugated at 12
3 and centrifugated at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min. Thereafter, the supernatants were harvested for the detection of Umb concentrations in lungs using UPLC-MS procedure with the chromatographic conditions as follows:
000g for 10 min. Thereafter, the supernatants were harvested for the detection of Umb concentrations in lungs using UPLC-MS procedure with the chromatographic conditions as follows:
Chromatographic analysis was performed on a Waters Acquity UPLC system (Waters Corp., Milford, MA, USA), consisting of a binary pump solvent management system, an online degasser, and an auto-sampler. An ACQUITY UPLC BEH C 18 (100 mm × 2.1 mm, 1.7 μm) column was applied for all analyses. And the column temperature was maintained at 35 °C. The mobile phase was composed of (A) formic acid aqueous solution (0.1%) and (B) acetonitrile using a gradient elution of 10–50% B at 0–4 min, 50–95% B at 4–6 min, 95% B at 6–8 min, 95–100% B at 8–9 min.
2.4 Induction of acute lung inflammation
Another 50 male BALB/c mice were randomly divided into five groups with 10 mice in each group: (1) control group, (2) LPS group, (3) LPS + dexamethasone (Dex, 2 mg kg−1) group, (4) LPS + Umb (20 mg kg−1) group, (5) LPS + Umb (Umb, 40 mg kg−1) group. The intratracheal instillation was done according to the method previously described with slight modifications.22 Briefly, the animals were intragastrically administered with the normal saline or drugs. 15 min after the treatment, mice were anesthetized with chloraldurate (3%), and then intratracheally administrated 20 μg of LPS dissolved in 50 μl phosphate buffered saline (PBS) to induce ALI. Simultaneously, control and LPS group were intratracheally given 50 μl PBS. 6 h later, all animals were sacrificed, bronchoalveolar lavage fluid (BALF) were harvested. The lung tissues were excised to immerse in 10% neutral-buffered formalin or −80 °C for pending test.2.5 Bronchoalveolar lavage
Six hours after intratracheal instillation of LPS, the animal was killed and the collection of bronchoalveolar lavage (n = 10) was performed three times through a tracheal cannula with 0.5 ml (total volume 1.5 ml) of autoclaved PBS to obtain the bronchoalveolar lavage fluid (BALF). BALF samples were centrifuged at 1500g for 10 min at 4 °C, the supernatants were stored in −80 °C for analysis of inflammatory cytokine concentrations with enzyme-linked immunosorbent assay (ELISA) kits (Biolegend, San Diego, USA). Then the pellets were resuspended in 100 μl of normal saline, centrifuged onto slides and stained for 8 min with Wright–Giemsa staining (Nanjing Jiancheng Bioengineering institute, Nanjing, China). The slides were quantified for neutrophils and total leukocyte by counting a total of 200 cells per slide at 40 magnification as the differential cell count.2.6 Lung wet-to-dry (W/D) weight ratios
The right lungs were removed at the end of the experiment. The trachea were separated from the lungs by blunt dissection, and the wet weight was determined. Subsequently, the lungs were incubated at 60 °C for 48 h to remove all moisture and dry weight of lungs were recorded. Finally, the lung W/D ratio was calculated.2.7 MPO activity in lung homogenates in BALF
The MPO activities in lung homogenates were determined using commercial test kits obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All procedures were according to the manufacturer's instructions. 100 mg lung tissues were homogenized with extraction buffer to acquire 5% homogenate. The enzymatic activity was determined using o-dianisidine as peroxidase substrates at 460 nm by a 96-well plate reader.2.8 Cytokine in BALF
BALF levels of IL-6, TNF-α, and IL-1β in BALF were measured by ELISA according to the manufacturer's instructions. The contents were calculated according to the standard curves.2.9 Histological assessment
In order to evaluate the histopathological changes of lung tissues, hematoxylin and eosin (H&E) staining was performed on paraffin embedded sections. The H&E staining process was carried out as previously described.23 Lung inflammatory cell count based on a five point scoring system was performed to estimate the severity of leukocyte infiltration. The scoring system was: 0: no cells, 1: a few cells, 2: a ring of cells with 1 cell layer deep, 3: a ring of cells with 2–4 cell layers deep; and 4: a ring of cells with more than 4 cell layers deep.2.10 Western blotting analysis
The lung tissues were homogenized, washed with PBS and incubated in lysis buffer in addition to a protease inhibitor cocktail (Sigma, St. Louis, MO) to obtain extracts of lung proteins. Protein concentrations were examined by BCA assay kit (Beyotime, Nanjing, China). The samples were separated on 10% sodium dodecyl sulphate polyacrylamide gels (SDS-PAGE) and were electrotransferred to polyvinylidene difluoride membranes (Millipore, MA, U.S.). Then, the blots were incubated with the appropriate concentration of specific primary antibodies. After washing, the blots were incubated with horseradish peroxidase-conjugated second antibody. Consequently, immunoreactivity was evaluated with an ECL plus Western blotting detection system (KeyGEN, Nanjing, China) and a Clinx ChemiScope chemiluminescence imaging system (Gel Catcher 2850, China). The membranes were stripped and reblotted with anti-GAPDH antibody by a ChemiScope analysis program.2.11 Statistical analysis
The results were expressed as means ± SDs. Comparison between groups was analyzed by one-way ANOVA with Tukey multiple test. P < 0.05 was taken as statistically significant.3. Results
3.1 Pharmacokinetic analysis of Umb concentration in lungs of LPS-induced ALI mice
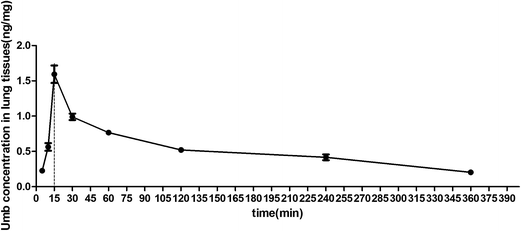
The pulmonary concentrations of Umb in LPS-induced lung injury mice were measured by UPLC, as shown in Fig. 1, the concentration of Umb in lungs were increased from 5 min. Notably, the Umb content reached the maximum at the 15 min time point. After that, the Umb concentration gradually decreased. The monitoring time-points data suggested that the maximal concentration of Umb in lung tissues might be reached at 15 min. Thus, we decided to performed the LPS stimulation 15 min after the drug administration.3.2 Effects of Umb on lung W/D ratio in lungs of LPS-induced ALI mice
To independently evaluate the effect of Umb on LPS-induced pulmonary edema, lung W/D weight ratios was assayed. Exposure to LPS for 6 h contributed to a significant increase in lung W/D ratio compared with that in control group (from 1.83 ± 0.25 to 6.23 ± 0.32 pg ml−1). As depicted in Fig. 2, Umb (20 mg kg−1, 40 mg kg−1) inhibited the severity of edema (to 4.09 ± 0.24, to 3.69 ± 0.19, respectively) when compared with that in the LPS group. Treatment with Dex (2 mg kg−1) also reduced the W/D ratio (to 3.04 ± 0.19). Our results displayed that significant reduction in the magnitude of the water content with the administration of Umb.3.3 Effects of Umb on the numbers of total leukocyte and neutrophils in BALF of LPS-induced ALI mice
To confirm the efficacy of LPS exposure, total leukocyte and neutrophil numbers in BALF were determined at the end of the experiment protocol. Instillation of LPS into the lungs produced a significant recruitment of total leukocyte neutrophils into the alveolar space. As revealed in Fig. 3, total leukocyte number (from 60.33 ± 5.03 to 162.67 ± 11.37) and neutrophil number (from 21.33 ± 2.52 to 88.33 ± 4.16) in BALF of LPS group significantly raised compared with those in control group. However, Umb (20 mg kg−1, 40 mg kg−1) could reduce the numbers of total leukocyte cell (to 127.67 ± 4.04, to 116.33 ± 6.51, respectively) and neutrophil (to 73.33 ± 4.04, to 61.67 ± 4.16, respectively). After Dex treatment, total leukocyte cell number (to 82.00 ± 4.58) and neutrophil number (to 35.67 ± 4.16) in BALF were also decreased obviously. These data indicated that Umb could inhibit the inflammatory infiltration in BALF of LPS-induced ALI.3.4 Effect of Umb on lung MPO activity in LPS-induced ALI mice
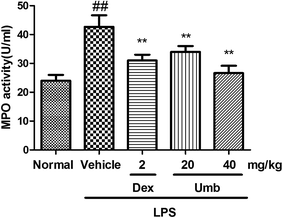
The MPO activity was examined to investigate the neutrophil accumulation in lung tissues. As revealed in Fig. 4, the activity of MPO increased after LPS administration (from 23.84 ± 1.43 to 43.05 ± 3.99 U ml−1). On the contrary, treatment with Umb (20 mg kg−1, 40 mg kg−1) markedly weakened the MPO activity (to 34.40 ± 1.70, to 26.78 ± 2.54 U ml−1, respectively). Besides, the administration of Dex (2 mg kg−1) also significantly decreased the MPO level (to 31.37 ± 1.82 U ml−1) in comparison with that in LPS group. These findings further suggested that Umb possessed ameliorative effect on the adhesion and migration of inflammatory cells in lungs.3.5 Effects of Umb on inflammatory cytokines in BALF of LPS-induced ALI mice
Cytokines play critical role in initiation, amplification, and perpetuation of inflammatory cascade in ALI. As shown in Fig. 5, in response to intratracheal administration of LPS, the levels of the cytokines IL-1β (from 10.27 ± 1.90 to 27.76 ± 3.54 pg ml−1), IL-6 (from 75.63 ± 3.63 to 139.10 ± 3.64 pg ml−1) and TNF-α (from 218.88 ± 8.64 to 688.57 ± 42.81 pg ml−1) were dramatically increased in BALF. On the other hand, Umb (20 mg kg−1, 40 mg kg−1) could down-regulate the IL-1β (to 17.63 ± 2.41, to 17.36 ± 2.94 pg ml−1, respectively), IL-6 (to 112.63 ± 6.06, to 105.94 ± 2.64 pg ml−1, respectively) and TNF-α (to 595.14 ± 28.78, to 514.86 ± 29.73 pg ml−1, respectively) levels. Moreover, Dex treatment also down-regulated the IL-1β (to 17.54 ± 2.90 pg ml−1), IL-6 (to 100.60 ± 3.92 pg ml−1) and TNF-α (to 395.06 ± 29.64 pg ml−1) levels in BALF.3.6 Effect of Umb on lung histology of LPS-induced ALI mice
The hematoxylin and eosin (H&E) observation was carried out to detect the protective effect of Umb on physiological impairment. In response to LPS stimulation, the sequestration and infiltration of neutrophil were observed around vessel and airway. On the contrary, the treatment group notably decreased the degree of inflammatory cell infiltration and alleviated the severity of histopathological alteration. The results indicated that Umb could attenuate the pathological inflammation in lung tissues of ALI-induced mice (Fig. 6).3.7 Effect of Umb on LPS-induced protein expression in mice of LPS-induced ALI mice
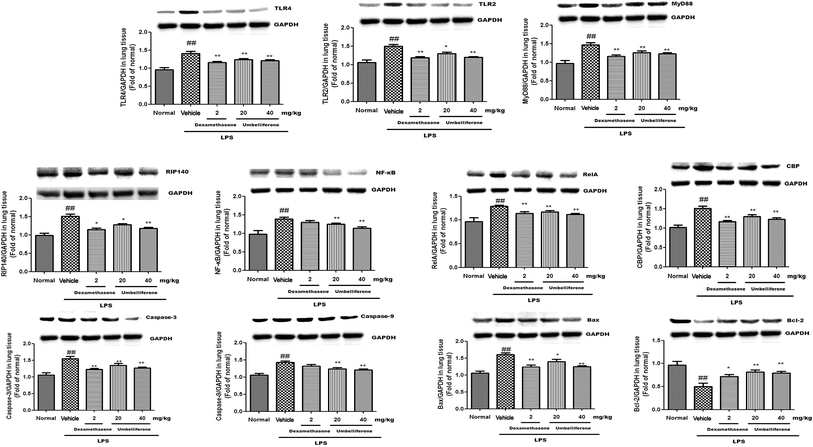
The expressions of inflammation and apoptosis-related protein TLR2, TLR4, MyD88, RIP140, RelA, CBP, NF-κB, caspase-3, caspase-9, Bax, and Bcl-2 were changed in lung tissues caused by LPS challenge. As shown in Fig. 7, the protein levels of TLR2 (to 1.51 fold), TLR4 (to 1.41 fold), MyD88 (to 1.47 fold), RIP140 (to 1.18 fold), RelA (to 1.37 fold), CBP (to 1.53 fold) and NF-κB (to 1.41 fold) in LPS group were significantly increased. Whereas the treatments with Umb (20 mg kg−1, 40 mg kg−1) effectively downregulated the expressions of TLR4 (to 1.24, 1.19 fold, respectively), MyD88 (to 1.23, 1.19 fold, respectively), RIP140 (to 1.27, 1.2 fold, respectively), RelA (to 1.23, 1.18 fold, respectively), CBP (to 1.34, 1.27 fold, respectively) and NF-κB (to 1.26, 1.19 fold, respectively). Administration of Dex (2 mg kg−1) also alleviated the expression of TLR2 (to 1.17 fold), TLR4 (to 1.15 fold), MyD88 (to 1.18 fold), RIP140 (to 1.18 fold), RelA (to 1.19 fold), CBP (to 1.21 fold) and NF-κB (to 1.32 fold).Additionally, stimulation with LPS contributed to the upregulations of caspase-3 (to 1.54 fold), caspase-9 (to 1.48 fold), Bax (to 1.57 fold) and the downregulation of Bcl-2 (to 0.51 fold). By contrast, Umb (20 mg kg−1, 40 mg kg−1) could downregulate the expressions of caspase-3 (to 1.32, 1.26 fold, respectively), caspase-9 (to 1.23, 1.2 fold, respectively), Bax (to 1.38, 1.23 fold, respectively) and upregulate the expression of Bcl-2 (to 0.83, 0.82 fold, respectively).
4. Discussion
The results of the present study indicated that the drug administration 15 min before LPS challenge might contribute to the better protective effect of Umb. The choice of the time point might be attributed to its maximum concentration which achieved at 15 min after the Umb treatment. The key features of LPS-induced acute lung injury are pulmonary edema, airspaces alveolar capillary barrier and the excessive neutrophils influx. Edema is the reliable index for both systemic and local inflammation.24 One of the most important process of the initial innate immune responses against bacterial infection is the vigorous recruitment of neutrophils. It is clear that the infiltration of neutrophils into lung tissues expresses multiple cytotoxic products and plays an principal role in acute lung injury.25 MPO is a major constituent of neutrophil cytoplasmic granules and serves as a direct indicator of neutrophil sequestration in lungs.26 In this research, we found that the treatment with Umb evidently reduced the W/D ratio, decreased MPO activity, inhibited the infiltration of total leukocyte and neutrophil in LPS-stimulated mice. Combined with the amelioration of histopathological changes, it was displayed that Umb could attenuate LPS-induced acute lung injury.LPS is known to induce the production of several inflammatory and chemotactic cytokines. IL-6, TNF-α, and IL-1β are characterized cytokines involved in the inflammatory process of ALI.27 These cytokines initiate, amplify, and perpetuate the inflammatory response.28 TNF-α is the earliest and primary endogenous mediator of inflammatory reaction.29 TNF-α, mainly produced by monocytes/macrophages, can trigger the inflammatory cascade, cause damage to the vascular endothelial cells and induce alveolar epithelial cells to produce other cellular factors including IL-6 and IL-1β.22 The data demonstrated that Umb significantly inhibited the overproductions of TNF-α, IL-6 and IL-1β in BALF caused by LPS challenge.
Toll-like receptor 4 (TLR4) is the major receptor of LPS and responsible for the recognition of Gram-negative bacteria. As its critical downstream molecule, myeloid differentiation factor 88 (MyD88) is promoted by the interaction of TLR4 with LPS.30 TLR2 can restore inflammatory homeostasis in bronchial epithelia.31 Former literature displayed that LPS triggered TLRs/MyD88 signaling to induce the inflammatory cascade.32 It was elicited that MyD88-dependent pathway was required for the infiltration of neutrophils and played an essential role in pulmonary inflammation.33 Our results demonstrated that Umb could obviously inhibit the expressions of TLR4, TLR2 and MyD88 in LPS-stimulated mice.
Nuclear receptor-interacting protein 1 (NRIP1), also known as receptor-interacting protein 140 (RIP140), is widely expressed and controls several physiological responses. RIP140 is involved in promotion of inflammatory processes by activating the generation of pro-inflammatory cytokines.34 RIP140 interacts with the nuclear factor light-chain enhancer of activated B cells (NF-κB) subunit RelA or cAMP-response element binding protein (CREB)-binding protein (CBP) to induce the generation of the inflammatory cytokines and apoptotic response.35 NF-κB controls the expression of genes involved in a number of physiological responses, such as immune reaction, acute-phase inflammatory responses, oxidative stress, cell adhesion, differentiation and apoptosis.36 NF-κB was also activated by TLRs/MyD88 pathway in acute lung injury.37 Previous investigators proved that NF-κB drove the inflammation and apoptosis in acute lung injury.38 Through blocking the RIP140/NF-κB pathway, Umb might simultaneously suppress the inflammatory and apoptosis effects in LPS-induced ALI.
Caspase-3 and caspase-9 play essential roles in the execution phase of cell apoptosis.39 The activation of caspase-3 is governed by a series of signaling transduction cascades, including the interaction between anti-apoptotic Bcl-2 and pro-apoptotic Bax proteins.40 Bcl-2 leads to cell survival by suppressing apoptosis while Bax shows the opposite effect. Bcl-2 is capable of forming a heterodimer with Bax, thus preventing Bax homodimerization and the sequential activation of caspase-3.41 Our data suggested that administration of Umb apparently inhibited the expressions of caspase-3, caspase-9, suppressed the pro-apoptotic Bax level and enhanced the anti-apoptotic Bcl-2 level in LPS-induced ALI.
In summary, pretreatment with Umb resulted in a significant reduction in the amount of inflammatory cells in BALF, lung W/D ratio and inflammatory cell infiltration into lung tissue. ELISA results indicated that Umb could significantly down-regulate TNF-α, IL-6 and IL-1β levels in BALF. Meanwhile, the protein expressions of TLR2, TLR4, MyD88, RIP140, RelA, CBP, NF-κB, caspase-3, caspase-9, Bax and Bcl-2 were effectively attenuated by the Umb pretreatment in the LPS-induced ALI. These results suggested that Umb exhibited a protective effect on LPS-induced ALI, possibly through suppressing pulmonary apoptosis and inflammation. Thus, Umb may be a potential therapeutic reagent that can be used to prevent ALI in the future. Further studies should be implemented to before clinical application.
Author contribution
Fen Luo performed the experiment, analyzed the data and wrote the paper. Rui Zhou performed the experiment and analyzed the data. Hui Lei and Yi Mou detected the time–concentration curve of Umb on acute lung injury. Ping Zhang and Yi Sun help to revise the pathway-related discussion. Tong Chen designed the study and wrote the paper. Ling He designed, financially supported, performed the detection of time–concentration curve and helped revised the manuscript. Tianhua Yan financially supported the work and designed the study.Acknowledgements
The study was supported by National 12th Five year Plan ‘‘Major Scientific and Technological Special Project for Significant New Drugs Creation” (no. 2012ZX09504001-001), National Natural Science Foundation of China (NSFC no. 81673434) and National Twelve Five Major Drug Discovery Project (no. 2011ZX09102-002-01).References
- T. Chen, Q. Guo, H. Wang, H. Zhang, C. Wang, P. Zhang, S. Meng, Y. Li, H. Ji and T. Yan, Effects of esculetin on lipopolysaccharide (LPS)-induced acute lung injury via regulation of RhoA/Rho Kinase/NF-small ka, CyrillicB pathways in vivo and in vitro, Free Radical Res., 2015, 49, 1459–1468 CrossRef CAS PubMed.
- M. A. Matthay, G. A. Zimmerman, C. Esmon, J. Bhattacharya, B. Coller, C. M. Doerschuk, J. Floros, M. A. Gimbrone Jr, E. Hoffman, R. D. Hubmayr, M. Leppert, S. Matalon, R. Munford, P. Parsons, A. S. Slutsky, K. J. Tracey, P. Ward, D. B. Gail and A. L. Harabin, Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group, Am. J. Respir. Crit. Care Med., 2003, 167, 1027–1035 CrossRef PubMed.
- J. Wang, L. Xiao, L. Zhu, M. Hu, Q. Wang and T. Yan, The effect of synthetic salidroside on cytokines and airway inflammation of asthma induced by diisocyanate (TDI) in mice by regulating GATA3/T-bet, Inflammation, 2015, 38, 697–704 CrossRef CAS PubMed.
- T. Chen, L. Xiao, L. Zhu, S. Ma, T. Yan and H. Ji, Anti-Asthmatic Effects of Ginsenoside Rb1 in a Mouse Model of Allergic Asthma Through Relegating Th1/Th2, Inflammation, 2015, 38, 1814–1822 CrossRef CAS PubMed.
- T. Chen, Z. Ma, L. Zhu, W. Jiang, T. Wei, R. Zhou, F. Luo, K. Zhang, Q. Fu, C. Ma and T. Yan, Suppressing Receptor-Interacting Protein 140: a New Sight for Salidroside to Treat Cerebral Ischemia, Mol. Neurobiol., 2015 DOI:10.1007/s12035-015-9521-7.
- M. Rosell, E. Nevedomskaya, S. Stelloo, J. Nautiyal, A. Poliandri, J. H. Steel, L. F. Wessels, J. S. Carroll, M. G. Parker and W. Zwart, Complex formation and function of estrogen receptor alpha in transcription requires RIP140, Cancer Res., 2014, 74, 5469–5479 CrossRef CAS PubMed.
- I. Zschiedrich, U. Hardeland, A. Krones-Herzig, M. Berriel Diaz, A. Vegiopoulos, J. Muggenburg, D. Sombroek, T. G. Hofmann, R. Zawatzky, X. Yu, N. Gretz, M. Christian, R. White, M. G. Parker and S. Herzig, Coactivator function of RIP140 for NF kappaB/RelA-dependent cytokine gene expression, Blood, 2008, 112, 264–276 CrossRef CAS PubMed.
- J. Wang, Y. T. Liu, L. Xiao, L. Zhu, Q. Wang and T. Yan, Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway, Inflammation, 2014, 37, 2085–2090 CrossRef CAS PubMed.
- L. Zhu, J. Wang, T. Wei, J. Gao, H. He, X. Chang and T. Yan, Effects of Naringenin on inflammation in complete freund's adjuvant-induced arthritis by regulating Bax/Bcl-2 balance, Inflammation, 2015, 38, 245–251 CrossRef CAS PubMed.
- R. Lucas, A. D. Verin, S. M. Black and J. D. Catravas, Regulators of endothelial and epithelial barrier integrity and function in acute lung injury, Biochem. Pharmacol., 2009, 77, 1763–1772 CrossRef CAS PubMed.
- W. Tao, Q. Su, H. Wang, S. Guo, Y. Chen, J. Duan and S. Wang, Platycodin D attenuates acute lung injury by suppressing apoptosis and inflammation in vivo and in vitro, Int. Immunopharmacol., 2015, 27, 138–147 CrossRef CAS PubMed.
- K. Zhang, J. Liu, X. You, P. Kong, Y. Song, L. Cao, S. Yang, W. Wang, Q. Fu and Z. Ma, P2X7 as a new target for chrysophanol to treat lipopolysaccharide-induced depression in mice, Neurosci. Lett., 2016, 613, 60–65 CrossRef CAS PubMed.
- W. Jiang, F. Luo, Q. Lu, J. Liu, P. Li, X. Wang, Y. Fu, K. Hao, T. Yan and X. Ding, The protective effect of Trillin LPS-induced acute lung injury by the regulations of inflammation and oxidative state, Chem.-Biol. Interact., 2016, 243, 127–134 CrossRef CAS PubMed.
- J. A. Fielhaber, S. F. Carroll, A. B. Dydensborg, M. Shourian, A. Triantafillopoulos, S. Harel, S. N. Hussain, M. Bouchard, S. T. Qureshi and A. S. Kristof, Inhibition of mammalian target of rapamycin augments lipopolysaccharide-induced lung injury and apoptosis, J. Immunol., 2012, 188, 4535–4542 CrossRef CAS PubMed.
- M. J. Kim, M. O. Sim, H. I. Lee, J. R. Ham, K. I. Seo and M. K. Lee, Dietary umbelliferone attenuates alcohol-induced fatty liver via regulation of PPARalpha and SREBP-1c in rats, Alcohol, 2014, 48, 707–715 CrossRef CAS PubMed.
- S. R. Subramaniam and E. M. Ellis, Umbelliferone and esculetin protect against N-nitrosodiethylamine-induced hepatotoxicity in rats, Cell Biol. Int., 2016, 40, 761–769 CrossRef CAS PubMed.
- Q. Su, W. Tao, H. Wang, Y. Chen, H. Huang and G. Chen, Umbelliferone attenuates unpredictable chronic mild stress induced-insulin resistance in rats, IUBMB Life, 2016, 68, 403–409 CrossRef CAS PubMed.
- J. Naowaboot, N. Somparn, S. Saentaweesuk and P. Pannangpetch, Umbelliferone Improves an Impaired Glucose and Lipid Metabolism in High-Fat Diet/Streptozotocin-Induced Type 2 Diabetic Rats, Phytother. Res., 2015, 29, 1388–1395 CrossRef CAS PubMed.
- S. M. Yu, D. H. Hu and J. J. Zhang, Umbelliferone exhibits anticancer activity via the induction of apoptosis and cell cycle arrest in HepG2 hepatocellular carcinoma cells, Mol. Med. Rep., 2015, 12, 3869–3873 CAS.
- M. O. Sim, H. I. Lee, J. R. Ham, K. I. Seo, M. J. Kim and M. K. Lee, Anti-inflammatory and antioxidant effects of umbelliferone in chronic alcohol-fed rats, Nutr. Res. Pract., 2015, 9, 364–369 CrossRef PubMed.
- J. F. Vasconcelos, M. M. Teixeira, J. M. Barbosa-Filho, M. F. Agra, X. P. Nunes, A. M. Giulietti, R. Ribeiro-Dos-Santos and M. B. Soares, Effects of umbelliferone in a murine model of allergic airway inflammation, Eur. J. Pharmacol., 2009, 609, 126–131 CrossRef CAS PubMed.
- T. Chen, Y. Mou, J. Tan, L. Wei, Y. Qiao, T. Wei, P. Xiang, S. Peng, Y. Zhang, Z. Huang and H. Ji, The protective effect of CDDO-Me on lipopolysaccharide-induced acute lung injury in mice, Int. Immunopharmacol., 2015, 25, 55–64 CrossRef CAS PubMed.
- C. Ma, L. Zhu, J. Wang, H. He, X. Chang, J. Gao, W. Shumin and T. Yan, Anti-inflammatory effects of water extract of Taraxacum mongolicum hand.-Mazz on lipopolysaccharide-induced inflammation in acute lung injury by suppressing PI3K/Akt/mTOR signaling pathway, J. Ethnopharmacol., 2015, 168, 349–355 CrossRef PubMed.
- A. Li, Y. Liu, L. Zhai, L. Wang, Z. Lin and S. Wang, Activating Peroxisome Proliferator-Activated Receptors (PPARs): a New Sight for Chrysophanol to Treat Paraquat-Induced Lung Injury, Inflammation, 2016, 39, 928–937 CrossRef CAS PubMed.
- W. Jing, M. Chunhua and W. Shumin, Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-kappaB pathway in vivo and in vitro, Toxicol. Appl. Pharmacol., 2015, 285, 128–135 CrossRef CAS PubMed.
- T. Chen, R. Wang, W. Jiang, H. Wang, A. Xu, G. Lu, Y. Ren, Y. Xu, Y. Song, S. Yong, H. Ji and Z. Ma, Protective Effect of Astragaloside IV Against Paraquat-Induced Lung Injury in Mice by Suppressing Rho Signaling, Inflammation, 2016, 39, 483–492 CrossRef CAS PubMed.
- Z. Tianzhu and W. Shumin, Esculin Inhibits the Inflammation of LPS-Induced Acute Lung Injury in Mice Via Regulation of TLR/NF-kappaB Pathways, Inflammation, 2015, 38, 1529–1536 CrossRef PubMed.
- T. Chen, W. Jiang, H. Zhang, X. You, M. Liu, L. Wang, P. Xiang, L. Xu, D. Zheng, X. Zhang, H. Ji, K. Hao and T. Yan, Protective effect of trillin against ethanol-induced acute gastric lesions in an animal model, RSC Adv., 2016, 6, 20081–20088 RSC.
- X. Y. Deng, J. J. Chen, H. Y. Li, Z. Q. Ma, S. P. Ma and Q. Fu, Cardioprotective effects of timosaponin B II from Anemarrhenae asphodeloides Bge on isoproterenol-induced myocardial infarction in rats, Chem.-Biol. Interact., 2015, 240, 22–28 CrossRef CAS PubMed.
- P. Xiang, T. Chen, Y. Mou, H. Wu, P. Xie, G. Lu, X. Gong, Q. Hu, Y. Zhang and H. Ji, NZ suppresses TLR4/NF-kappaB signalings and NLRP3 inflammasome activation in LPS-induced RAW264.7 macrophages, Inflammation Res., 2015, 64, 799–808 CrossRef CAS PubMed.
- S. Banerjee, J. Ninkovic, J. Meng, U. Sharma, J. Ma, R. Charboneau and S. Roy, Morphine compromises bronchial epithelial TLR2/IL17R signaling crosstalk, necessary for lung IL17 homeostasis, Sci. Rep., 2015, 5, 11384 CrossRef CAS PubMed.
- E. A. Frank, M. E. Birch and J. S. Yadav, MyD88 mediates in vivo effector functions of alveolar macrophages in acute lung inflammatory responses to carbon nanotube exposure, Toxicol. Appl. Pharmacol., 2015, 288, 322–329 CrossRef CAS PubMed.
- Q. Jiang, M. Yi, Q. Guo, C. Wang, H. Wang, S. Meng, C. Liu, Y. Fu, H. Ji and T. Chen, Protective effects of polydatin on lipopolysaccharide-induced acute lung injury through TLR4-MyD88-NF-kappaB pathway, Int. Immunopharmacol., 2015, 29, 370–376 CrossRef CAS PubMed.
- J. Nautiyal, M. Christian and M. G. Parker, Distinct functions for RIP140 in development, inflammation, and metabolism, Trends Endocrinol. Metab., 2013, 24, 451–459 CrossRef CAS PubMed.
- W. Jiang, R. Zhou, P. Li, Y. Sun, Q. Lu, Y. Qiu, J. Wang, J. Liu, K. Hao and X. Ding, Protective effect of chrysophanol on LPS/d-GalN-induced hepatic injury through the RIP140/NF-[small kappa]B pathway, RSC Adv., 2016, 6, 38192–38200 RSC.
- X. Y. Deng, H. Y. Li, J. J. Chen, R. P. Li, R. Qu, Q. Fu and S. P. Ma, Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice, Behav. Brain Res., 2015, 291, 12–19 CrossRef CAS PubMed.
- T. Zhang, J. Wang, S. Wang and C. Ma, Timosaponin B-II inhibits lipopolysaccharide-induced acute lung toxicity via TLR/NF-kappaB pathway, Toxicol. Mech. Methods, 2015, 25, 665–671 CrossRef CAS PubMed.
- X. C. Bao, Y. Q. Fang, P. You, S. Zhang and J. Ma, Protective role of peroxisome proliferator-activated receptor beta/delta in acute lung injury induced by prolonged hyperbaric hyperoxia in rats, Respir. Physiol. Neurobiol., 2014, 199, 9–18 CrossRef CAS PubMed.
- L. Zhu, T. Wei, J. Gao, X. Chang, H. He, F. Luo, R. Zhou, C. Ma, Y. Liu and T. Yan, The cardioprotective effect of salidroside against myocardial ischemia reperfusion injury in rats by inhibiting apoptosis and inflammation, Apoptosis, 2015, 20, 1433–1443 CrossRef CAS PubMed.
- J. Fu, Y. Wang, J. Zhang, W. Wu, X. Chen and Y. Yang, Anti-inflammatory and anti-apoptotic effects of oxysophoridine on lipopolysaccharide-induced acute lung injury in mice, Am. J. Transl. Res., 2015, 7, 2672–2682 Search PubMed.
- J. M. Adams and S. Cory, Bcl-2-regulated apoptosis: mechanism and therapeutic potential, Curr. Opin. Immunol., 2007, 19, 488–496 CrossRef CAS PubMed.
Footnote |
| † Fen Luo and Rui Zhou contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |