Self-assembly behavior of gels composed of dibenzylidene sorbitol derivatives and poly(ethylene glycol)
Wei-Chi Lai*ab and
Yi-Chin Leea
aDepartment of Chemical and Materials Engineering, Tamkang University, No. 151, Yingzhuan Rd., Tamsui Dist., New Taipei City 25137, Taiwan. E-mail: wclai@mail.tku.edu.tw; Fax: +886-2-2620-9887; Tel: +886-2-2621-5656 ext. 3516
bEnergy and Opto-Electronic Materials Research Center, Tamkang University, No. 151, Yingzhuan Rd., Tamsui Dist., New Taipei City 25137, Taiwan
First published on 10th October 2016
Abstract
The molecular interactions, self-assembly mechanism, rheological properties and microstructure characteristics of the gels obtained from three different dibenzylidene sorbitol derivatives [1,3:2,4-dibenzylidene-D-sorbitol (DBS), 1,3:2,4-di(3,4-dimethylbenzylidene)sorbitol (DMDBS) and 1,2,3-trideoxy-4,6:5,7-bis-O-[(4-propylphenyl)methylene]-nonitol (TBPMN)] and low-molecular-weight poly(ethylene glycol) (PEG) were investigated in this study. Ultraviolet/visible spectroscopy, Fourier transform infrared spectroscopy and molecular simulation calculation results demonstrated that the DBS/PEG systems had the strongest intermolecular hydrogen bonds and the most stable CH–π interactions, which exhibited T-shaped (point-to-face) orientations between DBS molecules; however, these strongest hydrogen bonds between DBS molecules did not improve the gel formation. We assume the interactions between DBS and PEG played an important role on the formation of the gels and influenced the gel formation time and gel formation/dissolution temperatures, as measured by dynamic rheological instruments. DMDBS and TBPMN have more alkyl groups that increased the steric hindrance to block the hydrogen bonding with PEG, which facilitated the self-assembly in comparison with DBS and led to larger diameters of the nanofibrils, as observed using scanning electron microscopy and transmission electron microscopy. In addition, the strongest intermolecular interactions between DBS molecules for the DBS/PEG systems led to the most regular structures of all the systems studied. This structure exhibited birefringent spherulitic-like textures under polarizing optical microscopy; the small-angle X-ray scattering results indicated a lamellar packing in these spherulitic-like morphologies.
1. Introduction
Dibenzylidene sorbitol is a low-molecular-weight amphiphilic molecule that is derived from a sugar alcohol. It is a butterfly shaped molecule that has hydrophilic hydroxyl groups and hydrophobic phenyl rings. The first synthesized dibenzylidene sorbitol was 1,3:2,4-dibenzylidene-D-sorbitol (DBS). Other chemically similar derivatives, such as 1,3:2,4-di(3,4-dimethylbenzylidene)sorbitol (DMDBS), 1,3:2,4-di-p-methylbenzylidene sorbitol (MDBS), and 1,2,3-trideoxy-4,6:5,7-bis-O-[(4-propylphenyl)methylene]-nonitol (TBPMN), are continually produced. Initially, dibenzylidene sorbitol derivatives were often used as nucleating agents in semicrystalline polymers, such as polyethylene and polypropylene, to change their nucleation rates and optical properties.1–3 In recent years, the discovery of molecules that are able to gel (the so-called “gelators”) has attracted a great deal of interest.4–8 They are dissolved at very low concentrations in organic solvents and liquid polymers to form gels, which result from the formation of 3-D nanofibrillar networks. The minimum nanofibrillar diameter is approximately 10–20 nm, as observed using electron microscopy. These gels have a large variety of applications including personal care products, battery electrolytes, template synthesis and drug-delivery systems.9–11Due to their interesting self-assembly behavior, many studies focused on the rheological properties and microstructures of the gels prepared from these dibenzylidene sorbitol derivatives in a variety of polar and non-polar organic solvents and liquid polymers.12–16 These studies found that the self-assembly behaviors of the gels were significantly influenced by the solvent or polymer matrix. For example, Yamasaki et al.13 showed that the solvent polarity has a large effect on the microstructures of the DBS gels. The self-assembly behavior of DBS gels was affected not only by the DBS concentrations but also by the chemical structures of the matrix media. Similarly, Liu et al.14 studied the solvent effects during DBS gel formation as well as the dynamic rheological properties of the gels. The researchers showed that the solvent polarity influenced the nanofibrillar diameters and gel dissolution temperatures. Several studies have demonstrated that different environmental conditions can influence the fibrillar structures and dimensions. For example, Lai et al.15 found that the fibrillar dimensions of DMDBS can be tuned by altering the heat treatment used. Chen et al.16 showed that the geometric confinement effects can change the fibrillar structures.
Despite the number of studies on individual compounds, comparisons of the self-assembly behaviors among the different dibenzylidene sorbitol derivatives are seldom reported. Watase et al.17 compared the gel formation behaviors of DBS in ethylene glycol with 1,3:2,4:5,6-tri-O-benzylidene-D-sorbitol (TBS) and 2,4-(mono)-O-benzylidene-D-sorbitol (MBS). Of the three molecules studied, TBS has the greatest number of benzylidene rings, whereas MBS possesses the most hydroxyl groups. In terms of gel formation ability, these derivatives are ranked in the following order: DBS, TBS and MBS. DBS molecules have rigid, chiral structures with two equatorial benzylidene rings and hydroxyl groups, and therefore they form gels much more easily. In addition, both hydroxyl groups and phenyl groups play an important role in the formation of the gels. Fahrlander et al.18 investigated a series of dibenzylidene sorbitol derivatives (MDBS, ethyl DBS (EDBS) and DMDBS) in which the phenyl rings were substituted with different alkyl groups in low-molecular-weight poly(propylene glycol) (PPG). The addition of methyl or ethyl groups to the phenyl rings reduces the polarity of the compounds, and therefore decreases their compatibility with polar PPG. The above-mentioned references were reported much earlier. In this study, the most used commercial sorbitol derivatives (DBS, DMDBS and TBPMN) from Milliken Chemical Company were chosen for the comparison of the different structures. The chemical structures of these compounds are shown in Fig. 1. From our results, using DMDBS and TBPMN, which have structure with more alkyl groups, increased the steric hindrance to block the hydrogen bonding with poly(ethylene glycol) (PEG) and led to larger nanofibrils dimensions than those obtained using DBS, which has not been previously reported.
Additionally, the self-assembly (gel formation) mechanism of the dibenzylidene sorbitol derivatives is discussed in this study. Yamasaki et al.19 found that pendant hydroxyl groups tend to form intramolecular hydrogen bonds and are also likely to have intermolecular interactions with the surrounding solvent. The terminal hydroxyl groups are able to participate in intermolecular hydrogen bonds and govern the gel formation. Moreover, the results indicated that the mechanism of gel formation, which could involve more than one type of molecular interaction, is complex. Wilder et al.20 suggested that the DBS molecules may prefer to orient the T-shaped interactions between phenyl rings with the same chiral orientation, in contrast with the results of Watase et al.,17 who proposed that DBS molecules stack on top of each other. In this study, we used experimental methods (UV/visible and IR spectroscopy) and molecular simulation calculations to investigate the molecular interactions, such as hydrogen bonding and π interactions, among these dibenzylidene sorbitol derivatives.
In this study, PEG was chosen as the matrix. PEG is hydrophilic and biocompatible and is often used in biomedical applications, including as a surfactant and dispersing agent.21–23 PEG also has a good potential for use in the fields of energy storage materials and battery electrolytes due to its high thermal and chemical stability.24,25 Our previous study has shown that the addition of DBS clearly improved the thermal and viscoelastic properties of the DBS/PEG gel electrolytes in dye-sensitized solar cells.10 Moreover, the conductivity of the prepared gel electrolytes was approximately that of the liquid electrolytes without DBS. In this study, gels composed of different sorbitol derivatives and PEG were prepared and compared for the first time. We believe that these systems may also have the potential for use in applications such as gel electrolytes.
2. Experimental
2.1. Materials
1,3:2,4-Dibenzylidene-D-sorbitol (DBS), 1,3:2,4-di(3,4-dimethylbenzylidene)sorbitol (DMDBS) and 1,2,3-trideoxy-4,6:5,7-bis-O-[(4-propylphenyl)methylene]-nonitol (TBPMN) were purchased from Milliken Chemical Company. The purity DBS, DMDBS and TBPMN was above 98%. Poly(ethylene glycol) (PEG) with a weight-average molecular weight of 400 g mol−1 was obtained from Acros.2.2. Sample preparation
The DBS/PEG, DMDBS/PEG and TBPMN/PEG samples were prepared by dissolving various amounts of DBS, DMDBS and TBPMN in PEG at 160 °C on a hot plate, under constant agitation. After the DBS, DMDBS and TBPMN were completely dissolved in PEG, the clear solutions were removed from the plate and cooled to room temperature. The samples were stored at 25 °C for a week, prior to conducting the experiments.2.3. Ultraviolet/visible (UV/visible) spectroscopy
The molecular interactions of the samples were determined using Ultraviolet/visible (UV/visible) spectroscopy (Thermo Evolution 300 UV/vis spectrophotometer) in a wavelength range between 200 and 700 nm at a scan rate of 120 nm min−1. The samples were completely dissolved at 160 °C on a hot plate, loaded into the detection cells and cooled to room temperature.2.4. Fourier transform infrared (IR) spectroscopy
The molecular interactions of the samples were measured by Fourier transform infrared (FTIR) spectroscopy (Nicolet Magna-IR 550 spectrometer). The samples were completely dissolved at 160 °C on a hot plate, poured onto KBr plates and cooled to room temperature. The neat samples of DBS, DMDBS and TBPMN were prepared by mixing the pure powder of each compound with KBr and pelleting the mixture.2.5. Molecular simulation of dimers
The 3-D molecular modeling of sample compounds was performed with the ChemBio 3D Ultra software (version 11). The distances at which the intermolecular hydrogen bonds appeared between molecules of DBS, DMDBS and TBPMN were calculated based on minimum energy conformations using geometry optimization.2.6. Dynamic rheological measurement
The gel formation time (tf) and temperature (Tf) of samples were measured using a rheometer (Anton Paar Physica MCR 101 rheometer). The samples were completely dissolved at 160 °C on a hot plate and rapidly poured into the cylinder of a rheometer that was maintained at 160 °C. Note that the 160 °C as the upper temperature is chosen because this temperature is above the gel dissolution temperature. The values of tf and Tf are defined as the start point where G′ became larger than G′′ at a cooling rate of 2 °C min−1. The strain amplitude was 0.5% and the frequency was maintained at 10 rad s−1. The gel dissolution temperatures (Td) of the samples were measured using a rheometer (Anton Paar Physica MCR 101 rheometer). The samples were completely dissolved at 160 °C on a hot plate, poured between the parallel-plates of a rheometer and cooled to room temperature. The values of Td were derived from the rheological data, as the intersection of the tangents from low to high temperatures, for the plot of G′ versus temperature with a heating rate of 5 °C min−1. The strain amplitude was 0.5%, and the frequency was maintained at 10 rad s−1. The parameters were as follows: a parallel-plate geometry with plates with a diameter of 20 mm and a gap size of 1 mm and a cylinder geometry with 10 mm inner diameter.2.7. Polarized optical microscopy (POM)
The morphologies of the samples were observed with a Precisa polarized optical microscope (Olympus CX 41) equipped with a camera. The solutions were treated at 160 °C to erase the thermal history, poured onto a glass plate, and covered with a cover slip. The samples were cooled to room temperature, and the POM images were taken.2.8. Small-angle X-ray scattering (SAXS) measurements
The self-assembled structures of the samples were probed by SAXS. The samples were introduced into the sample cell comprised of two Kapton windows. SAXS measurements were performed using an SAXS instrument (Bruker Nanostar). The X-ray source, a 1.5 kW X-ray generator (Kristalloflex 760) equipped with a rotating anode Cu target, was operated at 35 mA and 40 kV. The intensity profiles were output as the plots of the scattering intensity (I) versus the scattering vector, q = 4π/λ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(θ/2) (θ = scattering angle). The scattering intensities were corrected by the empty beam scattering, as well as the sensitivity of each pixel of the area detector.
sin(θ/2) (θ = scattering angle). The scattering intensities were corrected by the empty beam scattering, as well as the sensitivity of each pixel of the area detector.
2.9. Scanning electron microscopy (SEM)
The microstructures of the samples were observed using a field emission scanning electron microscope (Electronenmikroskopie GmbH Leo-1530). The samples were completely dissolved at 160 °C on a hot plate, and the solutions were rapidly poured onto a glass plate to form the gels. The gel samples were then placed in a vacuum oven at 60 °C for 12 h.2.10. Transmission electron microscopy (TEM)
The microstructures of samples were observed using a field emission transmission electron microscope (JEOL JEM-1230). The samples were completely dissolved at 160 °C on a hot plate, and the solutions were rapidly poured onto carbon-supported TEM grids to form the gels. The gel samples were then placed in a vacuum oven at 60 °C for 12 h.3. Results and discussion
3.1. Phase behavior
First, we investigated the phase behavior of three different dibenzylidene sorbitol derivative (DBS, DMDBS and TBPMN)/PEG systems by visual observation. All of the samples were stored at 25 °C for one week before observation. By visual observation, the different structures among these three systems were confirmed to have different phase behaviors. The gel formation for the DBS/PEG systems occurred when the DBS amount reached 3 wt%; however, for the DMDBS/PEG and TBPMN/PEG systems, the gel formed when the amount of DMDBS or TBPMN exceeded 0.5 wt%. Here, the “gel” is qualitatively observed by the naked eye. If we invert a vial, it can be seen that the solution does not flow due to the high viscosity, which we termed the “gel”. The later dynamic rheological experiments were used to characterize the viscoelasticity of these gels. In this study, for the discussion of the structures and properties of the samples in the gel or solution states, mixtures of DBS/PEG with 3 wt% DBS, DMDBS/PEG with 1 wt% DMDBS and TBPMN/PEG with 1 wt% TBPMN were chosen for the comparison of the gel states; mixtures of DBS/PEG with 1 wt% DBS, DMDBS/PEG with 0.5 wt% DMDBS and TBPMN/PEG with 0.5 wt% TBPMN were chosen for comparing the solution states. Note that both DMDBS/PEG and TBPMN/PEG gel systems were transparent, though not as clear as the DBS/PEG systems were, which could be due to the larger aggregation of the DMDBS and TBPMN molecules.When the amounts of TBPMN, DMDBS and DBS were higher than 1.5, 2 and 6 wt%, respectively, the phase separation occurred. The “phase separation” means that the samples exhibited a heterogeneous state (turbid) and the precipitation occurred. The reasons for the differences in the amounts that caused precipitation will be discussed in the next section.
3.2. Molecular interaction
Studies19,20 have found that the molecular interactions governed the self-assembly mechanism of these dibenzylidene sorbitol derivatives. The results of these studies indicated that the intermolecular interactions, such as the hydrogen bonding and π interactions between the phenyl rings, caused the dibenzylidene sorbitol derivatives to self-organize into a nanofibrillar network, which leads to the formation of gels. In this study, we used experimental methods (UV/visible and IR spectroscopy) and molecular simulation calculations to elucidate the self-assembly behavior of these systems.Fig. 2 shows the UV/visible spectra of the DBS/PEG, DMDBS/PEG and TBPMN/PEG systems in the solution and gel states. The peaks that range from 200 to 300 nm are usually characteristic of the phenyl groups. The ref. 26–28 note that the red shifts in the phenyl groups indicated that the π interactions occurred, and the transition was from the unstable π–π interactions to stable CH–π interactions. For example, because benzene has no substituents, the π interactions exhibited the most stable CH–π interactions with T-shaped (point-to-face) orientations between the benzene molecules.28–30 Multiple substituents on the benzene ring could increase the interaction energy, which changed the most stable point-to-face orientations to the edge-to-face orientations.31
 | ||
| Fig. 2 UV/visible spectra of the (a) DBS/PEG, (b) DMDBS/PEG and (c) TBPMN/PEG systems in the solution and gel states. The inset is enlarged spectra between 280 and 350 nm. | ||
As seen in Fig. 2, for the DBS/PEG and DMDBS/PEG systems, when the gels formed, the peak shifted to a longer wavelength (red shift), which implies the appearance of the π interactions. For the DBS/PEG systems, there is no substituent on the phenyl rings between DBS molecules; therefore, we assumed that the most stable point-to-face orientation was preferred, which was similarly suggested by Wilder et al.20 For the DMDBS/PEG systems, there are two methyl groups on the phenyl groups between DMDBS molecules; therefore, we assumed that the intermolecular geometries and interaction energies in the phenyl rings increased, which led to the possible edge-to-face orientations.
However, for the TBPMN/PEG systems, the peak position did not significantly change after the formation of the gels. The π interactions between TBPMN molecules were not clearly observed. The TBPMN molecules, which have more alkyl groups, increased the steric hindrance to block the π stacking between phenyl rings. In addition, the gels still formed in the TBPMN/PEG systems although the π interactions seemed absent. Therefore, we found that the hydrogen bonding played a more important role on the formation of the gels.
FTIR was used to investigate the hydrogen bonding among the neat dibenzylidene sorbitol derivatives and these derivatives in PEG. Fig. 3 shows the FTIR spectra of the neat DBS, DMDBS and TBPMN samples. From the IR handbook,32 the intermolecular hydrogen bonds for the OH groups appear in the range of 3200–3550 cm−1, and the intramolecular hydrogen bonds appear in the range of 3400–3590 cm−1. As seen in Fig. 3, two clear maximum peaks at approximately 3200–3300 cm−1 for the OH groups were found for all three samples, which could be attributed to the intermolecular hydrogen bonding. This indicated that these dibenzylidene sorbitol derivatives can self-assemble (aggregate) mainly through intermolecular hydrogen bonding. For the neat DBS samples, the maximum peaks have the highest intensity, and no shoulder peak was observed. In comparison, for the neat DMDBS and TBPMN samples, shoulder peaks appeared at approximately 3500–3600 cm−1 (indicated by the arrow), which could be attributed to intramolecular hydrogen bonds. The increased steric hindrance of the DMDBS and TBPMN molecules, which is due to the greater number of alkyl groups, may reduce the intermolecular hydrogen bonding, leading to the increased possibility of the intramolecular hydrogen bonding.
Fig. 4 shows the FTIR spectra of the DBS/PEG, DMDBS/PEG and TBPMN/PEG systems in the solution and gel states. Due to the large number of OH groups in PEG, a broad peak that ranged from 3100 to 3700 cm−1 was observed, which implied that the PEG has both the intra- and intermolecular hydrogen bonding. Moreover, this peak is overlapped by the OH groups for the DBS, DMDBS and TBPMN, which made it difficult to distinguish the OH peaks among the DBS/PEG, DMDBS/PEG and TBPMN/PEG systems.
 | ||
| Fig. 4 (a) FTIR spectra of the DBS/PEG gel and solution states, DMDBS/PEG gels, TBPMN/PEG gels and neat PEG and (b) enlarged FTIR spectra from 3000 to 4000 cm−1. | ||
In addition, as seen in Fig. 4(b), the intensity of the absorption peak that ranged from 3100 to 3700 cm−1 clearly increased with the increasing amount of DBS for the DBS/PEG systems. The ref. 19 reported that the pendant hydroxyl group of DBS tends to form intramolecular hydrogen bonds and is also likely to form intermolecular interactions with the surrounding solvents. The terminal hydroxyl group of DBS is able to form intermolecular hydrogen bonds between DBS molecules. When the DBS concentrations increased, the pendant hydroxyl group (the C5 position in Fig. 1(a)) of DBS tended to form intermolecular interactions with the surrounding solvent, PEG, and thus weakened the intramolecular hydrogen bonds with the neighboring acetal oxygen group (the C4 position in Fig. 1(a)). Therefore, the intermolecular hydrogen bonding between DBS and PEG increased. On the other hand, the terminal hydroxyl group (the C6 position in Fig. 1(a)) of DBS was likely to form intermolecular hydrogen bonds with an adjacent DBS molecule when the DBS concentrations increased. Thus, the intermolecular hydrogen bonding between DBS molecules increased. Both factors could cause the clear increase in intensity of the absorption peak that ranged from 3100 to 3700 cm−1.
For the comparison of the different dibenzylidene sorbitol derivatives in PEG, the greater number of alkyl groups in the DMDBS and TBPMN systems increased the steric hindrance, which likely caused the pendant hydroxyl groups (the C5 and C8 positions in Fig. 1(b) and (c)) of DMDBS and TBPMN to form intramolecular hydrogen bonds with the neighboring acetal oxygen groups (the C4 and C7 positions in Fig. 1(b) and (c)). Therefore, the increase in intramolecular hydrogen bonds decreased the intermolecular interactions with PEG for the DMDBS and TBPMN systems. In contrast, the lower steric hindrance of the DBS systems decreased the intramolecular hydrogen bonding, which led to a greater probability of reacting with PEG. Thus, the DBS systems were the most influenced by the presence of the PEG. In addition, at a fixed 1 wt% of the dibenzylidene sorbitol derivatives in PEG, only the DBS/PEG systems exhibited the solution state although the DBS systems may have the strongest intermolecular hydrogen bonds between DBS molecules. Therefore, we assume that the interactions between DBS and the solvent (PEG) played an important role in the formation of the gels.
3.3. Molecular simulation of dimers
Due to the complex IR spectra, which consist of overlapping peaks for the OH groups, a 3D molecular simulation model was used to elucidate the hydrogen bonding in these systems. Fig. 5 shows the assumed aggregate structures of the DBS, DMDBS and TBPMN dimers from the 3D molecular simulation model. The ChemBio 3D Ultra software (version 11) was used in this study. Here, the arrangement of the π interactions was obtained from our UV results. The position of the intermolecular hydrogen bonds was according to the ref. 19, which noted that the intermolecular hydrogen bonding likely occurred between the terminal hydroxyl group (the C6, C6 and C9 positions in Fig. 1(a)–(c)) of a DBS, DMDBS and TBPMN molecule and an acetal oxygen of an adjacent DBS, DMDBS and TBPMN molecule. The pendant hydroxyl group (the C5, C5 and C8 positions in Fig. 1(a)–(c)) of the DBS, DMDBS and TBPMN molecules formed intermolecular interactions with PEG. All of the structures were assumed to be in the minimum energy. For the TBPMN molecules, due to the smaller effect of the π interactions, only intermolecular hydrogen bonding was considered. As seen in Fig. 5, the distance for the appearance of the intermolecular hydrogen bonds between the DBS, DMDBS and TBPMN dimers (blue dashed line) were calculated to be approximately 1.622, 2.103, and 2.236 Å, respectively. Therefore, the calculated values confirmed that the DBS systems have the strongest intermolecular hydrogen bonds between DBS molecules. In comparison, the DMDBS and TBPMN systems, which have more sterically hindered structures, have fewer intermolecular hydrogen bonds between their respective sorbitol derivative molecules. | ||
| Fig. 5 Assumed aggregate structures of (a) DBS, (b) DMDBS and (c) TBPMN dimers obtained from the 3D molecular simulation model. | ||
To summarize the above results, due to the lower steric hindrance, the terminal hydroxyl group (the C6 position in Fig. 1(a)) of DBS formed the strongest intermolecular hydrogen bonds between DBS molecules. Moreover, the low steric hindrance also resulted in the lowest probability of intramolecular hydrogen bond formation for DBS, which led to the pendant hydroxyl group (the position C5 in Fig. 1(a)) of DBS forming the strongest intermolecular hydrogen bonds between DBS molecules and PEG in comparison with DMDBS and TBPMN. Therefore, DBS systems have the strongest intermolecular hydrogen bonds between DBS molecules and the strongest intermolecular hydrogen bonds between DBS and PEG among these systems.
3.4. Rheological measurement
We used dynamic rheological experiments to characterize the viscoelasticity of the DBS, DMDBS and TBPMN in PEG. First, for the DBS/PEG with 3 wt% DBS, DMDBS/PEG with 1 wt% DMDBS and TBPMN/PEG with 1 wt% TBPMN systems, the elastic modulus (G′) did not depend upon the frequency (ω), and G′ was greater than the elastic modulus (G′′) at all values of ω. Therefore, these samples can be considered gels. Fig. 6(a) shows the dynamic rheological data (G′ vs. time) of these DBS/PEG, DMDBS/PEG and TBPMN/PEG gel systems at a cooling rate of 2 °C min−1. Because the formation of these gels can not be suppressed during cooling, it was difficult to measure the gel formation time at the ambient temperature in the thermal equilibrium state. Therefore, in this study, the dynamic analysis (non-equilibrium method) was used. In addition, as mentioned in the ref. 33, the thermal properties, such as gel dissolution and formation temperatures, were not significantly influenced by the different heating/cooling rates. Therefore, the rheological measurements in this paper were conducted using a cooling rate of 2 °C min−1, the same as that in the ref. 33. The gel formation time (tf) and temperature (Tf) are defined as the start point where G′ became larger than G′′ (indicated by the arrows in Fig. 6(a)–(c)). The data obtained by rheological analysis are listed in Table 1. As seen in Table 1, the DBS/PEG gel systems exhibited the longest tf and lowest Tf, which indicated that the self-assembly (gel formation) behavior of DBS was the most hindered. We believed the self-assembly mechanism (gel formation) of DBS was controlled by the two competing factors. One is the intermolecular hydrogen bonding between DBS molecules, and the other is the intermolecular hydrogen bonding between DBS and the surrounding solvent. According to our analysis of the molecular interactions, the latter is a much more dominant factor for the self-assembly behavior of DBS. Thus, DBS interacted with PEG via the strongest intermolecular hydrogen bonding, which hindered the self-assembly ability of the DBS molecules. In contrast, the higher number of alkyl groups in sorbitol derivatives of the DMDBS/PEG and TBPMN/PEG systems increased the steric hindrance in their structures and decreased the intermolecular hydrogen bonding with PEG; therefore, both systems formed gels much more readily.| Samples | tf (min) | Tf (°C) | Td (°C) |
|---|---|---|---|
| DBS/PEG gel | 47.5 | 25.0 | 75.1 |
| DMDBS/PEG gel | 37.9 | 44.2 | 104 |
| TBPMN/PEG gel | 19.1 | 81.8 | 106 |
Fig. 6(d) demonstrates the dynamic rheological data (G′ vs. temperature) of the DBS/PEG, DMDBS/PEG and TBPMN/PEG gel systems at a heating rate of 5 °C min−1. The values for the gel dissolution temperature (Td) were derived as the intersection of the tangents from low to high temperatures.34 The data obtained by the rheological analysis are listed in Table 1. Fahrlander et al.18 showed that the gel dissolution and gel formation temperature is a first order transition. The first order transition temperature is described by Ttr = Htr/Str, where Htr and Str are the enthalpy and entropy of the phase transition, respectively. Htr is the much more dominant factor that influences Td and Tf.6 As seen in Table 1, the DBS/PEG systems had the lowest Td and Tf. As mentioned above, the interactions between DBS and PEG lead to a decrease in the self-assembly ability of DBS. Therefore, the enthalpy of gel dissolution and gel formation of the DBS/PEG systems decreased, and Td and Tf were the lowest in comparison with the DMDBS/PEG and TBPMN/PEG systems. In addition, the slight decrease in G′ in DMDBS/PEG and TMPMN/PEG systems may be related to higher crosslinking density of the samples. Due to the stronger self-assembly for the two systems, the aggregated structures may somewhat remain after the gel dissolution temperature.
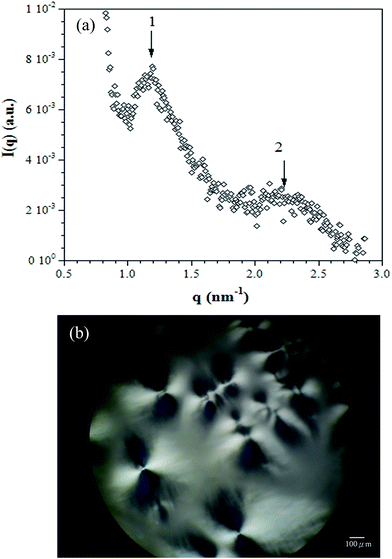
3.5. Microstructure: SAXS and POM
Fig. 7 shows the (a) SAXS spectra and (b) POM micrographs of the DBS/PEG with 3 wt% DBS (gel) system at room temperature. As seen in Fig. 7(a), SAXS results showed the presence of harmonic peaks in the spectra, which indicated that the DBS/PEG system has long-range regular structures. Moreover, the SAXS data exhibited a series of maximum peaks with a position ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, indicating that lamellar structures were formed. However, no peaks appeared in DMDBS/PEG and TBPMN/PEG systems. The structures of both the DMDBS/PEG and TBPMN/PEG systems were less regular than that of the DBS/PEG systems. Additionally, as seen in Fig. 7(b), birefringent spherulite-like textures were observed in the DBS/PEG system. The formation of these structures could be caused by the ordered lamellae. However, no birefringent morphology was found in either the DMDBS/PEG or TBPMN/PEG systems. We assumed that the strongest intermolecular interactions (hydrogen bonding) between DBS molecules in the DBS/PEG systems led to the more regular structure compared with the DMDBS/PEG and TBPMN/PEG systems. Therefore, the DBS/PEG systems showed the spherulitic-like texture, as observed by POM, and the regular lamellar structures, as measured by SAXS.
2, indicating that lamellar structures were formed. However, no peaks appeared in DMDBS/PEG and TBPMN/PEG systems. The structures of both the DMDBS/PEG and TBPMN/PEG systems were less regular than that of the DBS/PEG systems. Additionally, as seen in Fig. 7(b), birefringent spherulite-like textures were observed in the DBS/PEG system. The formation of these structures could be caused by the ordered lamellae. However, no birefringent morphology was found in either the DMDBS/PEG or TBPMN/PEG systems. We assumed that the strongest intermolecular interactions (hydrogen bonding) between DBS molecules in the DBS/PEG systems led to the more regular structure compared with the DMDBS/PEG and TBPMN/PEG systems. Therefore, the DBS/PEG systems showed the spherulitic-like texture, as observed by POM, and the regular lamellar structures, as measured by SAXS.
3.6. Microstructure: SEM and TEM
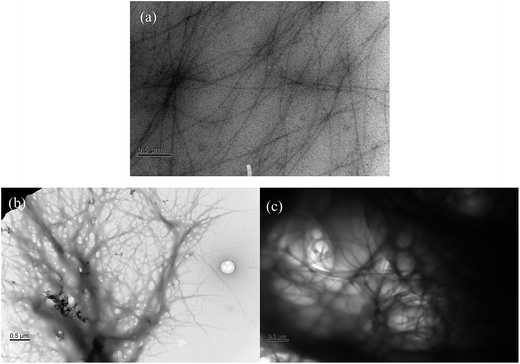
Fig. 8 shows the SEM micrographs of the DBS/PEG, DMDBS/PEG and TBPMN/PEG gel systems. For the DBS/PEG systems, the DBS aggregated structures were not easily observed due to the interferences from the formation of the spherulitic-like morphologies (see Fig. 7(b)). For the DMDBS/PEG and TBPMN/PEG systems, the typical aggregated structures (nanofibrils) were found, which is consistent with similar observations reported in the literature.4–8 Moreover, these nanofibrils formed a three dimensional network structure, leading to the gel behavior of the samples. However, as seen in Fig. 8(b) and (c), most fibrils were found to be aggregated, and the aggregated sizes were greater than 100 nm in diameter. SEM is usually used for the observation of the top surface of the structures. Later, TEM helps to confirm these aggregated structures.Fig. 9 shows the TEM micrographs of the DBS/PEG, DMDBS/PEG and TBPMN/PEG gel systems. For the DBS/PEG systems, the nanofibrils were observed, and their diameters were relatively uniform (approximately 10 nm). For the DMDBS/PEG systems, the diameters of the nanofibrils varied and ranged from 10 to 500 nm. For the TBPMN/PEG systems, the nanofibril diameters were also not uniform; the minimum diameter was approximately 10 nm, and the maximum was as large as 1 μm. The TBPMN/PEG systems, which have the structures with the most alkyl groups, have the highest steric hindrance to interfere with the intermolecular hydrogen bonding with PEG, which facilitates the self-assembly ability of TBPMN. Therefore, TBPMN had the greatest amount of aggregation, which led to the largest nanofibril diameters. In addition, due to the largest amounts of aggregated TBPMN and the largest nanofibril diameters, the TBPMN/PEG systems had the lowest amount of precipitation.
4. Conclusions
The molecular interactions (π interactions and hydrogen bonding), rheological properties and microstructures of the dibenzylidene sorbitol derivatives in PEG were discussed in this study. From the UV/visible results, the π interactions that exhibited the most stable point-to-face orientations between the phenyl rings were found in the DBS/PEG systems. For the DMDBS/PEG systems, the edge-to-face orientations could be arranged from the π interactions. However, no π interactions appeared in the TBPMN/PEG systems; nevertheless, the gels still formed. Therefore, the hydrogen bonding played an important role on the formation of the gels. From the IR results and molecular simulation calculations, DBS has the strongest intermolecular hydrogen bonds between DBS molecules, due to their lower steric hindrance. Moreover, DBS also has the strongest intermolecular hydrogen bonds between DBS and the solvent matrix (PEG) in comparison with DMDBS and TBPMN. In addition, the self-assembly (gel formation) mechanism of the dibenzylidene sorbitol derivatives was mainly controlled by the intermolecular hydrogen bonding between the dibenzylidene sorbitol derivative and the solvent. Furthermore, due to the strongest intermolecular hydrogen bonds between DBS and PEG, the DBS/PEG systems had the slowest gel formation time and the lowest gel formation/dissolution temperatures, as determined using dynamic rheological instruments. As for the observation of the microstructures, the strongest intermolecular hydrogen bonds between DBS molecules in the DBS/PEG systems led to the most regular structures. The DMDBS/PEG and TBPMN/PEG systems both have more alkyl groups than DBS, which increased their steric hindrance to decrease the hydrogen bonding with PEG, therefore facilitating the self-assembly ability and leading to the larger nanofibril diameters, as observed by SEM and TEM.Acknowledgements
We gratefully acknowledge financial support from the Ministry of Science and Technology of Taiwan.References
- M. Kristiansen, M. Werner, T. Tervoort and P. Smith, Macromolecules, 2003, 36, 5150–5156 CrossRef CAS.
- K. Wang, C. J. Zhou, C. Y. Tang, Q. Zhang, R. N. Du, Q. Fu and L. Li, Polymer, 2009, 50, 696–706 CrossRef CAS.
- J. Cao, K. Wang, W. Cao, Q. Zhang, R. Du and Q. Fu, J. Appl. Polym. Sci., 2009, 112, 1104–1113 CrossRef CAS.
- W.-C. Lai, S.-C. Tseng, S.-H. Tung, Y.-E. Huang and S. R. Raghavan, J. Phys. Chem. B, 2009, 113, 8026–8030 CrossRef CAS PubMed.
- K. K. Diehn, H. Oh, R. Hashemipour, R. G. Weiss and S. R. Raghavan, Soft Matter, 2014, 10, 2632–2640 RSC.
- W.-C. Lai, S.-J. Tseng and Y.-S. Chao, Langmuir, 2011, 27, 12630–12635 CrossRef CAS PubMed.
- H. Oh, N. Yaraghi and S. R. Raghavan, Langmuir, 2015, 31, 5259–5264 CrossRef CAS PubMed.
- D. J. Cornwell, O. J. Daubney and D. K. Smith, J. Am. Chem. Soc., 2015, 137, 15486–15492 CrossRef CAS PubMed.
- T. Schamper, M. Jablon, M. H. Randhawa, A. Senatore and J. D. Warren, J. Soc. Cosmet. Chem., 1986, 37, 225–231 CAS.
- W.-C. Lai and C.-C. Chen, Soft Matter, 2014, 10, 312–319 RSC.
- E. J. Howe, B. O. Okesola and D. K. Smith, Chem. Commun., 2015, 51, 7451–7454 RSC.
- M. Watase and H. Itagaki, Bull. Chem. Soc. Jpn., 1998, 71, 1457–1466 CrossRef CAS.
- S. Yamasaki and H. Tsutsumi, Bull. Chem. Soc. Jpn., 1995, 68, 123–127 CrossRef CAS.
- S. Liu, W. Yu and C. Zhou, Soft Matter, 2013, 9, 864–874 RSC.
- W.-C. Lai, S.-J. Tseng and P.-H. Huang, J. Nanopart. Res., 2015, 17, 456 CrossRef.
- W. Chen, Y. Yang, C. H. Lee and A. Q. Shen, Langmuir, 2008, 24, 10432–10436 CrossRef CAS PubMed.
- M. Watase, Y. Nakatani and H. Itagaki, J. Phys. Chem. B, 1999, 103, 2366–2373 CrossRef CAS.
- M. Fahrlander, K. Fuchs and C. Friedrich, J. Rheol., 2000, 44, 1103–1108 CrossRef CAS.
- S. Yamasaki, Y. Ohashi, H. Tsutsumi and K. Tsujii, Bull. Chem. Soc. Jpn., 1995, 68, 146–151 CrossRef CAS.
- E. A. Wilder, R. J. Spontak and C. K. Hall, Mol. Phys., 2003, 101, 3017–3021 CrossRef CAS.
- H. C. Kim, H. Lee, J. Khetan and Y.-Y. Won, Langmuir, 2015, 31, 13821–13833 CrossRef CAS PubMed.
- E. Schneck, I. Berts, A. Halperin, J. Daillant and G. Fragneto, Biomaterials, 2015, 46, 95–104 CrossRef CAS PubMed.
- S. Yadav, S. R. Deka, G. Verma, A. K. Sharma and P. Kumar, RSC Adv., 2016, 6, 8103–8117 RSC.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- S. Karaman, A. Karaipekli, A. Sari and A. Bicer, Sol. Energy Mater. Sol. Cells, 2011, 95, 1647–1653 CrossRef CAS.
- H. Tsutsumi and S. Yamasaki, Bull. Chem. Soc. Jpn., 1996, 69, 561–564 CrossRef.
- H. Yao, K. Domoto, T. Isohashi and K. Kimura, Langmuir, 2005, 21, 1067–1073 CrossRef CAS PubMed.
- C. A. Janiak, Dalton Trans., 2000, 21, 3885–3896 RSC.
- M. O. Sinnokrot, E. F. Valeev and C. D. Sherrill, J. Am. Chem. Soc., 2002, 36, 10887–10893 CrossRef.
- M. O. Sinnokrot, A. L. Ringer, R. P. Lively and C. D. Sherrill, Chem.–Eur. J., 2006, 12, 3821–3828 CrossRef PubMed.
- B. M. Farrell, W. B. Jennings and J. F. Malone, Acc. Chem. Res., 2001, 34, 885–894 CrossRef.
- G. Socrates, Infrared and Raman characteristic group frequencies: tables and charts, Wiley, Chichester, U.K., 2001 Search PubMed.
- E. A. Wilder, C. K. Hall and R. J. Spontak, J. Colloid Interface Sci., 2003, 267, 509–518 CrossRef CAS PubMed.
- M. Fahrländer, K. Fuchs and C. Friedrich, J. Rheol., 2000, 44, 1103–1119 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |