Preparation of AlN microspheres/UHMWPE composites for insulating thermal conductors
Abstract
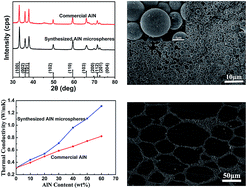
AlN microspheres were prepared by applying both sol–gel technique and gas reduction nitridation, and then modified by 3-aminopropyltriethoxysilane (KH550) to increase the affinity with the polymer. The KH550 modified AlN microspheres/UHMWPE composites were fabricated by solution mixing and hot pressing technique. Their microstructure, thermal stability, thermal conductivity and dielectric properties were investigated. The AlN microspheres/UHMWPE composite demonstrated much higher thermal conductivity in comparison with the commercial AlN particles/UHMWPE composite. The dielectric constant and dielectric loss of the KH550 modified AlN microspheres/UHMWPE composites increased slightly with increasing AlN content. The KH550 modified AlN microspheres/UHMWPE composites still had a high surface resistivity (>1014 Ω m) and volume resistivity (>1015 Ω m).

 Please wait while we load your content...
Please wait while we load your content...