Sensitive detection of tumor cells based on aptamer recognition and isothermal exponential amplification†
Wei Tanga,
Ting Zhanga,
Qinggui Li*b,
Hui Wanga,
Honghong Wanga and
Zhengping Li*a
aKey Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education, Key Laboratory of Analytical Chemistry for Life Science of Shaanxi Province, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710062, Shaanxi Province, P. R. China. E-mail: lzpbd@snnu.edu.cn
bDepartment of Orthopaedics, Affiliated Hospital of Hebei University, Baoding 071002, P. R. China. E-mail: 2268345154@qq.com
First published on 16th September 2016
Abstract
The noninvasive detection of tumor cells is significantly important for early diagnosis of cancers and monitoring of their progress. Herein, we have developed a novel aptamer-based isothermal exponential amplification reaction (EXPAR) for sensitive detection of tumor cells. In this new assay, biotinylated sgc8c DNA aptamers are immobilized on streptavidin-coated magnetic beads. After incubation with target CCRF-CEM cells and magnetic isolation, the tumor cells are detected by translating the structure-switching of an aptamer upon tumor cell binding into an input of DNA trigger for EXPAR. Since more and more specific aptamers towards different tumor cells would be identified in the future, our strategy can be easily extended to the detection of different tumor cells just by simply altering the corresponding aptamers. Therefore, this EXPAR-based strategy may serve as a generic platform for the accurate detection of tumor cells, which has great potential for the early diagnosis of cancers.
Introduction
The noninvasive detection of tumor cells is significantly important for early diagnosis of cancers and monitoring of their progress.1,2 Currently, immunoassays have been widely employed for the detection of tumor cells based on the specific interaction between antibodies and the surface markers of tumor cells.3 However, the immunoassays have generally shown some drawbacks, such as insufficient sensitivity, complex manufacture progress, long assay times and expensive cost. What is more, the identification of effective surface markers targeting a specific kind of tumor cells remains a challenge with current technologies.4 So far, only very few biomarkers can be used for detection of specific tumor cells, which greatly limits the application scope of the immunoassays. As an alternative to antibodies, DNA aptamers have been recently selected to recognize different tumor cells by using an in vitro technique known as SELEX (systematic evolution of ligands by exponential enrichment),5–7 which have exhibited distinct advantages, such as easy synthesis and chemical modification, long-term stability and lack of immune response.8 Most importantly, the selected aptamers have shown high binding affinity, cell specificity and can be screened out without prior knowledge about the surface biomarkers of a specific type of tumor cells, showing great potential for sensitive and selective detection of tumor cells.Recently, significant efforts have been devoted to development of aptamer-based assays for tumor cell detection with typically imaging and flow cytometry techniques by measurement of fluorescence,9–11 surface-enhanced Raman scattering (SERS),12 chemiluminescence and magnetic relaxation.13,14 These methods have provided important insights into the specific interaction between the selected aptamers and tumor cells and realized highly sensitive detection of the tumor cells. However, the imaging analysis and flow cytometry analysis generally need specially trained personnel for sample tagging and instrument operation and expensive instrumentation, which is poorly suited for the routine and point-of-care detection in common clinical laboratory. Therefore, aptamer-based assays for specific detection of tumor cells by using common instruments available in ordinary laboratory have received considerable attention, such as fluorescence method,15 electrochemical method,16–18 and electrochemiluminescence assay.19 Most of these methods require aptamer-based signal amplification techniques in order to achieve high sensitivity for tumor cell detection, which generally suffer from costly labeled reagents, time-consuming operations, or sophisticated process.
Aptamers are single stranded DNA/RNA molecules that can fold by intramolecular interaction into unique three-dimensional conformations for target recognition.5 Highly sensitive detection and quantification of specific-sequence nucleic acids based on nucleic acid amplification techniques is one of the fastest growing areas in both biochemistry and clinical chemistry. The nucleic acid amplification is one of the most well-established technologies.20 Therefore, by translating the structure-switching of an aptamer upon tumor cell binding into input of DNA/RNA for nucleic acid amplification, nucleic acid amplification techniques should provide an ideal and powerful tool for highly sensitive detection of tumor cells in view of the specific interaction between aptamers and the tumor cells. Song et al. have developed an aptamer-based polymerase chain reaction (PCR) method for ultrasensitive tumor cell detection,21 in which the fluorescein-labeled dGTP and dUTP are employed to perform the PCR and the PCR products are detected through fluorescence resonance energy transfer (FRET) with a cationic conjugated polymer. Although the PCR-based method achieves high sensitivity, it needs expensive fluorescein-labeled reagents and the additional detection step after PCR amplification. Moreover, the PCR needs a temperature cycling protocol that limits the rate of the amplification and make it more complex, which is prone to false-negative results.
The limitations of PCR-based DNA/RNA assays have spurred the development of various isothermal amplification techniques.20,22 More recently, Zhang et al. have reported a colorimetric aptasensor for tumor cell detection based on nicking endonuclease-assisted isothermal amplification.23 However, the linear amplification mechanism of this method limits the sensitivity. Among the isothermal amplification techniques, the exponential amplification reaction (EXPAR) is a powerful tool for highly sensitive detection of short DNA with the simplest design, which enables 106 to 109-fold amplification of DNA trigger within a short time.24,25 In our previous study, EXPAR has been applied to highly sensitive detect microRNA,26 in which the presence of as little as 0.1 zmol of microRNA can be determined. Through combining activity of different enzymes and sensitive detection of the specifically produced DNA fragments, EXPAR has also used for detection of telomerase,27 methyltransferase,28 transcription factors,29 and gene-specific methylation.30 Aptamers are short oligonucleotides, which should be well-suited to initiate EXPAR. By using aptamer-based target-triggering EXPAR, Zhang et al. have developed a simple and sensitive method for detection of platelet-derived growth factor BB (PDGF-BB) through detection of DNA produced by specific interaction between the aptamer and PDGF-BB.31 In this work, taking the advantages of EXPAR for highly efficient signal amplification and the specific recognition of aptamers to tumor cells, we have developed a simple, robust and label-free method for tumor cell detection with a high sensitivity.
Experimental section
Materials and reagents
The streptavidin-coated magnetic beads (Dynabeads MyOne Streptavidin C1) were purchased from Invitrogen (Life technologies, U.S.A.). All of the DNA oligonucleotides were synthesized by TaKaRa (Dalian, China) and the sequences are listed in Table S1.† SYBR Green I (20× stock solution in dimethyl sulfoxide, 20 μg mL−1) was purchased from Xiamen Bio-Vision Biotechnology (Xiamen, China). Vent (exo-) DNA polymerase, Nt.BstNBI nicking endonuclease and dNTPs were obtained from New England Biolabs (U.S.A.). All of other reagents were of analytical reagent grade and used as purchased without further purification.Cell culture and counting
CCRF-CEM, HL-60 and K562 cells were purchased from cell culture center of Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Beijing, China). All of these cell lines were maintained in Roswell Park Memorial Institute 1640 (RPMI 1640) (Sigma, U.S.A.) supplemented with 10% fetal bovine serum (Hyclone, U.S.A.), 100 U mL−1 penicillin and 100 μg mL−1 streptomycin (Hyclone, U.S.A.), and incubated in the Forma Steri-Cycle CO2 incubators (Thermo Fisher Scientific, Inc., U.S.A.) at 37 °C with 5% CO2.For EXPAR analysis, the target cells were collected and centrifuged at 800 rpm for 5 min, resuspended in phosphate buffer saline (PBS) buffer, and then the cell densities were counted by a hemacytometer. The tumor cell numbers (10–1000) were calculated according to the series dilutions of such cell samples. During all experiments, the cells were kept in an ice bath at 4 °C to prevent cell lysis.
Standard procedures of EXPAR-based assay
Typically, after magnetic isolation, 1 μL dynabeads MyOne streptavidin C1 (Stv-MBs) were suspended in 2 μL 2 × binding and wash (B&W) buffer (10 mM Tris–HCl pH 7.5, 1 mM EDTA, 2 M NaCl). Afterward, 2 μL biotinylated sgc8c aptamers (500 nM) were added to such dispersion of Stv-MBs and incubated at room temperature for 30 min. The aptamer-functionalized MBs were washed 3 times by B&W buffer and then re-suspended in 2 μL 2× B&W buffer. After that, 2 μL cDNA (1 μM) was introduced to hybridize with sgc8c at 37 °C for 1 h. The prepared MB-aptamer–cDNA biocomplexes were washed 3 times by B&W buffer, and subsequently were suspended in 10 μL PBS containing different number of tumor cells. After incubation at 4 °C for 30 min, the MBs and cells were removed from the solution through magnetic isolation and centrifugation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for 10 min. The supernatant containing the released cDNA was collected, and finally 1 μL of such cDNA-containing supernatant was applied to subsequent EXPAR.
000 for 10 min. The supernatant containing the released cDNA was collected, and finally 1 μL of such cDNA-containing supernatant was applied to subsequent EXPAR.
The reaction mixtures of the EXPAR system were prepared separately on ice as part A and part B. Part A consisted of ThermoPol buffer, amplification Template I and Template II, dNTPs and cDNA target; part B consisted of Nt.BstNBI buffer, Nt.BstNBI nicking endonuclease, Vent (exo-) DNA polymerase and SYBR Green I. Part A and B were mixed immediately before being placed in the Stepone Real-Time PCR System (ABI, U.S.A.). The EXPAR was performed in a volume of 10 μL containing the cDNA target, Template I and Template II (both 0.1 μM), dNTPs (250 μM), Nt.BstNBI (0.5 U μL−1), Vent (exo-) DNA polymerase (0.04 U μL−1), SYBR Green I (0.4 μg mL−1), 1 × ThermoPol buffer (20 mM Tris–HCl, pH 8.8, 10 mM KCl, 10 mM (NH4)2SO4, 2 mM MgSO4, 0.1% Triton X-100), and 1 × Nt.BstNBI buffer (25 mM Tris–HCl, pH 7.9, 50 mM NaCl, 5 mM MgCl2, 0.5 mM DTT). The EXPAR was performed at 55 °C, and the real-time fluorescence signal was monitored at intervals of 1 min.
Affinities assay of FITC-labeled sgc8c with tumor cells by using fluorescence microscopy and flow cytometry
The binding affinity of sgc8c aptamers to different kinds of tumor cells was first investigated by fluorescence imaging analysis. Typically, 1 × 106 of each kind of tumor cells were respectively centrifuged at 800 rpm for 5 min and then incubated with 200 μL of binding buffer (5 mM MgCl2, 4.5 g L−1 glucose, 0.1 mg mL−1 yeast tRNA, 1 mg mL−1 BSA and 5% FBS in PBS) containing 50 nM FITC-labeled sgc8c at 4 °C for 30 min. Then such cells were washed twice with 500 μL binding buffer and resuspended in 200 μL of binding buffer. 10 μL of the cell suspension was dropped on a coverslip and the fluorescence images of the cells were measured on an Olympus FV1200-DP73 fluorescence microscope (Olympus, Japan).The binding affinity of sgc8c aptamers to different kinds of tumor cells were also examined by flow cytometry analysis. Briefly, in a total 200 μL of binding buffer, 5 × 105 of each type of cells were respectively incubated with 200 nM of FITC-labeled sgc8c at 4 °C for 30 min in the dark. Then the cells were washed by 500 μL binding buffer twice and resuspended in 300 μL of binding buffer. 200 μL of such cell suspension was subjected to flow cytometry analysis on a Guava easyCyte 8HT Flow Cytometry (Millipore, U.S.A.).
Results and discussion
Design principle of the proposed EXPAR – based assay
Fig. 1 illustrates the principle of our EXPAR-based assay for tumor cell detection by using sgc8c as a proof-of-concept aptamer. Firstly, the biotinylated sgc8c DNA aptamers which can specifically recognize CCRF-CEM cells are immobilized on the streptavidin-coated magnetic beads (Stv-MB).32,33 Then a short DNA (cDNA), which is complementary to the sequence of sgc8c at its 3′ terminus, hybridizes with sgc8c to form a MB-aptamer–cDNA complex. When the complexes are incubated with CCRF-CEM cells, sgc8c sequences will strongly bind on the surface of the tumor cells accompanied with rigid aptamer structure-switching.32,33 As a result, cDNA will be released into the solution. The number of released cDNA can faithfully reflect the amount of tumor cells, which can be quantitatively determined by the subsequent EXPAR. | ||
| Fig. 1 Schematic illustration of the EXPAR-based dual-template assay for the detection of tumor cells. | ||
In our EXPAR system, two amplification templates (Template I and II) are utilized to improve the efficiency of amplification. Template I contains three portions of functional sequences. The sequence at its 3′ terminus (dark red) is complementary to the cDNA, while the sequence of its 5′ terminus (green) is identical to those at both the 3′ and 5′ termini of Template II. So the Template II contains two repeat sequences at both the 3′ and 5′ termini. A specific sequence (3′-CTCAG-5′) in the middle (blue) of both Template I and Template II is the recognition site of Nt.BstNBI nicking endonuclease.
At first, the cell binding-released cDNA can hybridize with the complementary sequence at the 3′ terminus of Template I. Vent (exo-) DNA polymerase then catalyzes the extension reaction of the cDNA along Template I to form double-stranded DNA (dsDNA). Afterward, Nt.BstNBI nicking endonuclease recognizes the 3′-CTCAG-5′ site in the middle of the dsDNA and cleaves the upper DNA strand at a site four bases downstream. The cleaved DNA strand containing the recognition site will extend again, and thus the short single-stranded DNA (ssDNA) will be displaced and released due to the strand–displacement activity of Vent (exo-) DNA polymerase.24,25 In this regard, the cDNA can be linearly amplified by the repetition of extension, cleavage, and strand displacement to produce lots of ssDNA. Subsequently, the produced ssDNA can further hybridize with the sequence at the 3′ terminus of Template II. With the catalysis of Vent (exo-) DNA polymerase, the ssDNA will be extended along Template II to form a dsDNA, which can be cleaved with Nt.BstNBI nicking enzyme to release more and more ssDNA. The released ssDNA will hybridize with the sequence at the 3′ terminus of another Template II. As such, extension, cleavage, and strand displacement can be repeated continuously resulting in the exponential amplification under isothermal conditions to produce numerous ssDNA and dsDNA. Simultaneously, SYBR Green I is employed as the fluorescent dye for the real-time detection of the EXPAR products.
Optimization of the EXPAR conditions and analytical performance
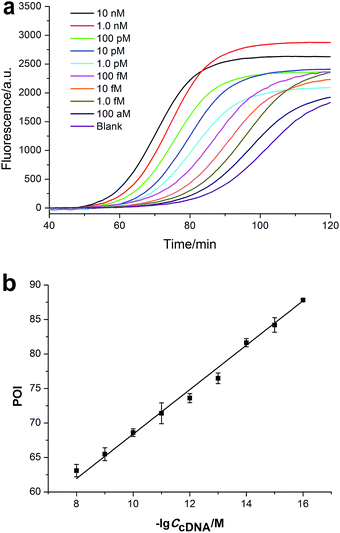
Prior to tumor cell detection, the cDNA was directly used as the trigger of EXPAR to optimize the experimental conditions such as the amount of DNA polymerase and nicking enzyme, the concentration of Template I, and the ratio between Template I and II for EXPAR (see Fig. S1–S4 in ESI†). Under the optimum conditions, the cDNA could be quantitatively detected from 100 aM to 10 nM through real-time measurement of the fluorescence signals of the EXPAR products (Fig. 2a). The point of inflection (POI), the time at which the fluorescence rises significantly above the background, is used for the quantitative detection of the cDNA. The POI values are linearly dependent on the logarithm (lg) of the concentration of the cDNA from 100 aM to 10 nM. The correlation equation is POI = 36.19–3.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg
lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CcDNA (M) with a correlation coefficient R = 0.9976 (Fig. 2b). Therefore, this EXPAR system has a high sensitivity across a wide dynamic range of eight orders of magnitude for the cDNA detection.
CcDNA (M) with a correlation coefficient R = 0.9976 (Fig. 2b). Therefore, this EXPAR system has a high sensitivity across a wide dynamic range of eight orders of magnitude for the cDNA detection.
Application of the EXPAR-based assay for detection of CCRF-CEM cells
The highly sensitive EXPAR assay was further applied to tumor cell detection by using sgc8c as the specific probe for recognizing CCRF-CEM cells following the procedures depicted in Fig. 1. Sgc8c was a recognized DNA aptamer for CCRF-CEM cells identified through cell-SELEX.28,29 Fig. 3a shows that our EXPAR-based assay can evidently detected CCRF-CEM from 1000 to 10 cells, and the POI values are linearly dependent on the logarithm (lg) of the cell number (Fig. 3b). The correlation equation is POI = 94.15–5.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg
lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ncell number (R = 0.9956). Therefore, this EXPAR-based dual-template assay offers a sensitive platform for the detection of tumor cells. With increasing aptamers identified for different tumor cells by cell-SELEX, we believe this method can be widely applied to the detection of various tumor cells by combining with different aptamers.
Ncell number (R = 0.9956). Therefore, this EXPAR-based dual-template assay offers a sensitive platform for the detection of tumor cells. With increasing aptamers identified for different tumor cells by cell-SELEX, we believe this method can be widely applied to the detection of various tumor cells by combining with different aptamers.
Specificity of the EXPAR-based assay
To investigate the specificity of the EXPAR-based assay, other two cell lines, HL-60 (acute myeloid leukemia, AML) and K562 (chronic myeloid leukemia, CML), were employed in this work. CCRF-CEM cells are classified to T-cell human acute lymphoblastic leukemia (T-ALL) cells. Most of AML cells are similar to T-ALL cells. Tan and co-workers have reported that sgc8c showed high affinity toward T-ALL cells and several AML cells.28,30 Through mass spectroscopy analysis and flow cytometry assay, they preliminarily found that a membrane protein tyrosine kinase 7 (PTK7) was the binding target of sgc8c. They pointed out the PTK7 was not expressed in normal mature T cells, whereas it was highly expressed in T-ALL cells and was also expressed in a subset of AML cells.32 As a typical AML cells, HL-60 was chose to study the interference from AML cells. K562 was employed as a negative control which could not bind to sgc8c basically. Compared with the sensitivity towards CCRF-CEM, the responses of 100 K562 cells cannot be discriminated from the blank signal (Fig. 4b), indicating the high specificity of the EXPAR-based assay for detection of CCRF-CEM cells. For HL-60 cells, the fluorescence responses of at least 50 cells can just be distinguished from blank signal (Fig. 4a) suggesting that HL-60 cells have a weak combination with sgc8c. This result is well consistent with the previous reports.34 To quantitatively assess the detection results, the relative detection was calculated by using the correlation equation shown in Fig. 3b, which is corresponding to cell numbers calculated by the POI values of the real-time fluorescence curves of 100 K562 or HL-60 cells. The relative detection showed that there is a slight interference from HL-60 (19.3%) cells and no interference from K562 cells (Fig. 4c).To further verify the affinity of sgc8c aptamers to the three kinds of cell lines, fluorescein (FITC)-labeled sgc8c aptamers were respectively incubated with CCRF-CEM, HL-60, and K562 cells and then such cells were all analyzed by fluorescence microscopy and flow cytometry. The bright fluorescence images of CCRF-CEM cells (Fig. 5b) indicate that they have a very high affinity to sgc8c, and the results of flow cytometry show about 90.6% of CCRF-CEM cells are stained by FITC-sgc8c (Fig. 5c). The flow cytometry result indicates that about 27.5% of HL-60 cells could combine with FITC-sgc8c (Fig. 5c), and accordingly much less HL-60 cells were stained by FITC-sgc8c (Fig. 5b) compared with CCRF-CEM cells. The results in Fig. 5 also reveal that K562 cells exhibit the lowest affinity to sgc8c because almost no fluorescence signal could be observed under the fluorescence microscope because only 2.25% of K562 cells combine with FITC-sgc8c according to the flow cytometry analysis. These results are in good accordance with those obtained by our EXPAR system. The specificity of the proposed EXPAR system is highly dependent on the specificity and affinity of the used aptamer to target cells.
Conclusions
In summary, we have proposed a robust strategy for the detection of cancer cells by combining aptamer-based specific cell recognition and EXPAR-based signal amplification. The strong affinity between the aptamer and target tumor cells renders the proposed method a high specificity for detection of specific kind of tumor cells. Furthermore, due to the high signal amplification efficiency of EXPAR, high sensitivity is achieved which enables accurate detection of 10 target cells. The comparison between the proposed EXPAR-based assay and other reported techniques for detection of tumor cells is listed in ESI as Table S2.† It can be seen from Table S2† that the proposed method can be characterized with simple operation and high sensitivity. Since more and more specific aptamers towards different tumor cells would be identified in the future, our assay can be easily extended for the detection of different tumor cells just by simply altering corresponding aptamers. Therefore, this EXPAR-based assay may serve as a generic platform for the accurate identification of tumor cells of low abundances in a bulk population of normal cells, which is of great potential for the early diagnosis of cancers.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21335005, 21472120), Program for Innovative Research Team in University (IRT_15R43), Program for Innovative Research Team in Shaanxi Province (No. 2014KCT-28), the Open Funds of the State Key Laboratory of Electroanalytical Chemistry (SKLEAC201409), and the Fundamental Research Funds for the Central Universities (GK201603042).Notes and references
- C. Jin, S. M. McFaul, S. P. Duffy, X. Deng, P. Tavassoli, P. C. Black and H. Ma, Lab Chip, 2014, 14, 32 RSC.
- P. Chen, Y. Y. Huang, K. Hoshino and X. Zhang, Lab Chip, 2014, 14, 446 RSC.
- K. Hoshino, Y. Y. Huang, N. Lane, M. Huebschman, J. W. Uhr, E. P. Frenkel and X. Zhang, Lab Chip, 2011, 11, 3449 RSC.
- Z. Tang, D. Shangguan, K. Wang, H. Shi, K. Sefah, P. Mallikratchy, H. W. Chen, Y. Li and W. Tan, Anal. Chem., 2007, 79, 4900 CrossRef CAS PubMed.
- D. Shangguan, Y. Li, Z. Tang, Z. C. Cao, H. W. Chen, P. Mallikaratchy, K. Sefah, C. J. Yang and W. Tan, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 11838 CrossRef CAS PubMed.
- K. Sefah, D. Shangguan, X. Xiong, M. B. O'Donoghue and W. Tan, Nat. Protoc., 2010, 5, 1169 CrossRef CAS PubMed.
- H. Kang, M. B. O'Donoghue, H. Liu and W. Tan, Chem. Commun., 2010, 46, 249 RSC.
- X. Fang and W. Tan, Acc. Chem. Res., 2010, 43, 48 CrossRef CAS PubMed.
- J. Yin, X. He, K. Wang, F. Xu, J. Shangguan, D. He and H. Shi, Anal. Chem., 2013, 85, 12011 CrossRef CAS PubMed.
- J. K. Herr, J. E. Smith, C. D. Medley, D. Shangguan and W. Tan, Anal. Chem., 2006, 78, 2918 CrossRef CAS PubMed.
- Y. F. Huang, H. T. Chang and W. Tan, Anal. Chem., 2008, 80, 567 CrossRef CAS PubMed.
- P. Wu, Y. Gao, Y. Lu, H. Zhang and C. Cai, Analyst, 2013, 138, 6501 RSC.
- S. Bi, B. Ji, Z. Zhang and S. Zhang, Chem. Commun., 2013, 49, 3452 RSC.
- S. Bamrungsap, T. Chen, M. I. Shukoor, Z. Chen, K. Sefah, Y. Chen and W. Tan, ACS Nano, 2012, 6, 3974 CrossRef CAS PubMed.
- C. D. Medley, S. Bamrungsap, W. Tan and J. E. Smith, Anal. Chem., 2011, 83, 727 CrossRef CAS PubMed.
- Z. Yi, X. Y. Li, Q. Gao, L. J. Tang and X. Chu, Analyst, 2013, 138, 2032 RSC.
- L. K. Kheyrabadi, M. A. Mehrgardi, E. Wiechec, A. P. F. Turner and A. Tiwari, Anal. Chem., 2014, 86, 4956 CrossRef PubMed.
- L. Qu, J. Xu, X. Tan, Z. Liu, L. Xu and R. Peng, ACS Appl. Mater. Interfaces, 2014, 6, 7309 CAS.
- G. Jie, L. Wang, J. Yuan and S. Zhang, Anal. Chem., 2011, 83, 3873 CrossRef CAS PubMed.
- L. Yan, J. Zhou, Y. Zheng, A. S. Gamson, B. T. Roembke, S. Nakayama and H. O. Sintim, Mol. BioSyst., 2014, 10, 970 RSC.
- J. Song, F. Lv, G. Yang, L. Liu, Q. Yang and S. Wang, Chem. Commun., 2012, 48, 7465 RSC.
- J. Kim and C. J. Easley, Bioanalysis, 2011, 3, 227 CrossRef CAS PubMed.
- X. Zhang, K. Xiao, L. Cheng, H. Chen, B. Liu, S. Zhang and J. Kong, Anal. Chem., 2014, 86, 5567 CrossRef CAS PubMed.
- J. Van Ness, L. K. Van Ness and D. J. Galas, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 4504 CrossRef CAS PubMed.
- E. Tan, B. Erwin, S. Dames, T. Ferguson, M. Buechel, B. Irvine, K. Voelkerding and A. Niemz, Biochemistry, 2008, 47, 9987 CrossRef CAS PubMed.
- H. Jia, Z. Li, C. Liu and Y. Cheng, Angew. Chem., Int. Ed., 2010, 122, 5630 CrossRef.
- Y. Zhang, L. J. Wang and C. Y. Zhang, Chem. Commun., 2014, 50, 1909 RSC.
- Q. Xue, L. Wang and W. Jiang, Chem. Commun., 2015, 51, 13538 RSC.
- Y. Zhang, J. Hu and C. Y. Zhang, Anal. Chem., 2012, 84, 9544 CAS.
- Y. Xu, C. Niu, X. Xiao, W. Zhu, Z. Dai and X. Zou, Anal. Chem., 2015, 87, 2945 CrossRef CAS PubMed.
- Z. Z. Zhang and C. Y. Zhang, Anal. Chem., 2012, 84, 1623 CrossRef CAS PubMed.
- D. Shangguan, Z. Cao, L. Meng, P. Mallikaratchy, K. Sefah, H. Wang, Y. Li and W. Tan, J. Proteome Res., 2008, 7, 2133 CrossRef CAS PubMed.
- M. You, L. Peng, N. Shao, L. Zhang, L. Qiu, C. Cui and W. Tan, J. Am. Chem. Soc., 2014, 136, 1256 CrossRef CAS PubMed.
- D. Shangguan, Z. Cao, Y. Li and W. Tan, Clin. Chem., 2007, 53, 1153 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The sequences of target DNA and primers used in the work, optimization of experimental conditions. See DOI: 10.1039/c6ra18225a |
| This journal is © The Royal Society of Chemistry 2016 |