New strategy for magnetic gas sensing
Abstract
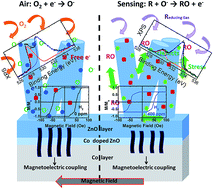
A new-concept approach to room temperature magnetic gas sensing has been developed, based on newly designed Co/ZnO hybrid nanostructures. The sensor prototype has been demonstrated to be sensitive, reversible, fast and scalable. In this work, the role of the Co/ZnO surface and interface in the gas-sensing mechanism has been clarified. In order to attain a comprehensive understanding of the physics governing the remarkable properties of the Co/ZnO stack, an extensive electronic and structural investigation has been carried out. The reaction at room temperature with target gases involves the surface and the lateral faces of the ZnO nanorods, with the formation of structural defects and vacancies. In particular, it has been discovered that the stress enhancement as well as the change in the polarizability of the ZnO nanorods are transduced by Co in a change of its magnetization. The interplay between these phenomena may provide versatile approaches to tune the intrinsic electronic, magnetic and optical properties of the hybrid nanostructure.


 Please wait while we load your content...
Please wait while we load your content...