Adsorption of radioactive iodine on surfactant-modified sodium niobate†
Abstract
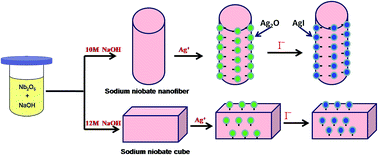
Iodine radioisotopes have been released into the environment both by the nuclear industry and as a result of medical treatment using radioactive materials, potentially posing a serious threat to human health. Thus, when developing applications of radioactive iodine, emergency procedures for dealing with it, and preventing its leakage, are constant concerns. In this study, nanofibers and cubes of Ag2O anchored sodium niobate composites were synthesized by a wet chemical process, and characterized by means of XRD, SEM, TEM and BET techniques. It was found that the well-dispersed Ag2O nanocrystals (3–5 nm) were firmly anchored on the external surface of sodium niobate along the planes with crystallographic similarity to those of Ag2O. These composites can efficiently capture I− anions by precipitating AgI, firmly attaching it to the substrate and allowing it to be recovered easily for safe disposal. Variations in the iodine removal abilities with differing pH value, adsorption time, adsorption temperature, initial I− concentration, and in the presence of competing ions, were studied. The maximum monolayer adsorption capacities of I− anions for Ag2O anchored sodium niobate nanofibers and cubes were found to be 296 and 193 m2 g−1 respectively. TEM, XRD, XPS and Raman results indicated that I− anion adsorption was mainly due to the Ag2O nanocrystals on the sodium niobate surfaces. We conclude that Ag2O deposited on sodium niobate hass a great potential for removal of radioactive iodine from waste water.

 Please wait while we load your content...
Please wait while we load your content...