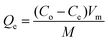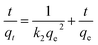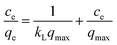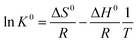Adsorption of Cu2+ and Cd2+ from aqueous solution by novel electrospun poly(vinyl alcohol)/graphene oxide nanofibers
Ping Tan,
Junjie Wen,
Yongyou Hu* and
Xiaojun Tan
Ministry of Education Key Laboratory of Pollution Control and Ecological Remediation for Industrial Agglomeration Area, College of Environment and Energy, South China University of Technology, Guangzhou 510006, PR China. E-mail: ppyyhu@scut.edu.cn; Fax: +86-20-39380508; Tel: +86-20-39380506
First published on 16th August 2016
Abstract
Novel poly(vinyl alcohol)/graphene oxide (PVA/GO) nanofibers were fabricated by electrospinning and applied to remove Cu2+ and Cd2+ from aqueous solution. The adsorption performances of the PVA/GO nanofibers were investigated by removing Cu2+ and Cd2+. The results showed that the adsorption of Cu2+ and Cd2+ onto PVA/GO nanofibers increased as the pH was increasing, but only slightly reduced with the increasing of ionic strength. The adsorption could reach equilibrium within 25 min and the experimental kinetic data followed the pseudo-second-order kinetic model. The equilibrium adsorption data can be well fitted with the Langmuir model. The thermodynamic parameters calculated from adsorption isotherms at four different temperatures indicated that the adsorption processes were endothermic and spontaneous. The PVA/GO nanofibers have good regeneration ability and can be recycled 8 times with a small amount of loss in adsorption efficiency. FTIR and XPS results indicated that the carboxyl and the carbonyl groups of GO on the surface of the nanofibers mainly participated in the adsorption of Cu2+ and Cd2+.
1. Introduction
Heavy metal pollution has been a serious worldwide problem due to its persistence, non-biodegradability, extreme toxicity and accumulation in biological tissues passing through the food chain.1,2 Adsorption is one of the most important and widely used technologies for the removal of heavy metals from wastewater due to its simplicity, economical feasibility and moderate operational conditions.3,4 Traditional adsorbents, such as zeolites,5 activated carbon6 and sugarcane bagasse,7 suffer from low adsorption capacities or poor removal efficiencies. Therefore, it is important to seek more effective adsorbents for heavy metal ions removal.Nanomaterials have attracted more and more attention due to their huge specific surface area and abundant active sites. So far, many nanomaterials as adsorbents have been applied to remediate contaminants from wastewater. Carbon-based nanomaterials are one type of the most promising nanomaterials and have been surveyed as adsorbents for the removal of organic pollutants and heavy metals, such as carbon nanotubes8 and graphene,9,10 and the excellent adsorption ability of these carbon nanomaterials are demonstrated.
Graphene oxide (GO), as a new development of carbon-based nanomaterial, has attracted increasing attention for potential use in the removal of heavy metals due to its huge specific surface area (approximately 2620 m2 g−1) and abundant oxygen-containing groups (epoxide, hydroxyl, carbonyl and carboxyl groups). GO has gained great success in removal heavy metal ions,11–14 e.g. Cd2+, Cu2+, Pb2+ and Co2+. However, GO in aqueous solution shows high dispersion performance owing to the existence of hydrophilic groups on its basal planes and edges.15 Thus, achieving separation of GO in aqueous solution after adsorption is very difficult. Fitting GO to a suitable solid support by covalent conjugation and self-assembly is favorable to promoting phase separation and enhancing adsorption efficiency. Unfortunately, covalent conjugation need not only complex synthesis process, but also use a lot of poisonous and harmful chemicals. In contrast, self-assembly process for assembling GO onto support is very fast and needs only a small amount of GO to form the monolayer on the surface of the support. Therefore, it is very necessary to use appropriate self-assembly technology to fabricate separable GO-based nanocomposites.
Electrospinning is a simple and versatile self-assembly technique for producing continuously multi-functional nanofibers, with ultrafine diameters, from a variety of polymers.16–20 Electrospinning have shown many application in sensors,21 catalysis,22 filter,23,24 drug delivery25,26 and tissue engineering.27,28 Recently, the application of electrospun nanofibers as adsorbents in the removal of heavy metal has attracted much attention, and different types of nanofibers have been fabricated by electrospinning to remove heavy metal ions from aqueous solution. In general, electrospinning precursor can be done by mixing adsorptive substrate with spinnable polymer and then be electrospun to nanofibrous adsorbents, such as wool keratose/silk fibroin nanofibers,29 poly(vinyl alcohol)/TiO2 nanofibers30 and poly(vinyl alcohol)/SiO2 nanofibers.31 GO, as a super adsorbing material, can evenly mix with many spinnable polymers due to its hydrophilic oxygen-containing groups. Therefore, GO based nanofibers should be able to be fabricated by electrospinning and were applied as adsorbents for the removal of heavy metal ions from wastewater. Additionally, poly(vinyl alcohol) (PVA) is a common spinnable polymer that is nontoxic and water-soluble at high temperatures.32 PVA acting as spinning aid is often spun into all kinds of functional nanofibers.30,31,33 Hence, it is of interest to fabricate GO/PVA nanofibers through electrospinning for the adsorption of heavy metal ions from wastewater. These GO/PVA nanofibers should have the following advantages: (1) the oxygen-containing functional groups on the surface of GO can form hydrogen bonds with hydroxyl groups of PVA chains, this may lead to an increase of GO loading in PVA nanofibers and prevent GO detachment from the PVA matrix, which are favorable to improving adsorption efficiency; (2) the spinning precursor is prepared by using water as solvent of PVA and GO, and the preparation process is simple and environmentally-friendly; (3) the anchoring of GO on PVA nanofibers could weaken the interaction between GO nanosheets and thus impede the stacking of GO nanosheets. Notably, PVA nanofibers incorporating a little of GO have been explored as reinforcing materials. However, the use of these materials for the removal of heavy metal ions has never been reported, especially for the nanofibers with large amounts of GO.
In this study, PVA/GO nanofibers (the weight ratio of GO/PVA is as large as possible) were prepared by electrospinning and applied as adsorbents for the adsorption of typical heavy metal ions, such as Cu2+ and Cd2+. The adsorption properties of the nanofibers toward Cu2+ or Cd2+ in aqueous solution were studied under various conditions, such as pH, temperature, contact time, ionic strength and metal ion concentration as well as the regeneration ability. Besides, the adsorption mechanisms of Cu2+ or Cd2+on the PVA/GO nanofibers were elucidated according to Fourier transformed infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS).
2. Experimental
2.1. Materials
Graphite powder was supplied from Sigma-Aldrich. Poly(vinyl alcohol) with a degree of polymerization of 1700 and an alcoholysis degree of 99 mol% was provided by Aladdin Industrial, Inc. All other chemicals used in the experiments were analytical grade products.2.2. Electrospinning of PVA/GO composite nanofibers
GO was prepared by a modified Hummers method34 and freeze drying approach as reported in our previous work.35 The fabrication procedure for PVA/GO composite nanofibers was as follows: first, different PVA/GO suspensions with GO loadings of 0–45 wt% with respect to PVA content (8 wt%) were used for electrospinning to optimize the spinnability of PVA/GO composite nanofibers. Preliminary results showed that PVA/GO suspensions with GO loadings of 0–40 wt% with respect to PVA content (8 wt%) avoided blockage of the spinning nozzle and yielded uniform and bead-free PVA/GO nanofibers. When the load of GO exceed 40 wt%, blockage of the spinning nozzle would occur and PVA/GO nanofibers were not formed. Therefore, a 40 wt% GO concentration with respect to PVA content (8 wt%) was considered to be optimal. Fig. 1 describes an electrospinning process that can incorporate GO into continuous PVA nanofibers. The specific procedure for electrospinning of PVA/GO nanofibers with GO loadings of 40 wt% is as follows: PVA powder (12 g) was added into distilled water and magnetically stirred at 92 °C for 3 h to obtain a 12 wt% homogeneous solution. A certain quality of GO was added to distilled water and sonicated for 1 h. Then, the GO suspension was mixed with 10 mL of PVA aqueous solution. The mixture was stirred for 12 h and sonicated for an additional 30 min to obtain the uniform precursor suspension. The prepared solution was then placed into a 10 mL syringe equipping with a 0.9 mm inner diameter nozzle. The nozzle connected high-voltage power supply acted as an electrode, the collector covered with aluminum foil served as the counter electrode. The electrospinning process parameters were performed at an applied voltage of 32 kV, a feeding rate of 1 mL h−1 and a needle tip-to-collector distance of 15 cm. All electrospinning preparations were conducted at room temperature (25 °C) and the relative humidity was maintained at 45% by a dehumidifier/steam humidifier.2.3. Characterization methods
X-ray diffraction (XRD) patterns of the samples were obtained using a D8 Advance (Bruker) X-ray diffractometer equipped with a Cu-Kα radiation source (λ = 1.5418 Å). Scanning electron microscope (SEM) was taken on a FEI Sirion 200 scanning electron microscope. Fourier-transform infrared spectroscopy (FT-IR) of KBr pressed pellets was performed using a Nicolet 5700 spectrometer. Raman spectra were recorded with a Renishew inVia Raman spectrometer. Transmission electron microscope (TEM) images were acquired on a Tecnai G2 F20S-TWIN with an operating voltage of 200 kV. The X-ray photoelectron spectroscopy (XPS) measurements were conducted with an ESCA Lab 220I-XL system.2.4. Adsorption and desorption experiments
A certain quality of PVA/GO nanofibers and different concentrations of metal solution were placed in polyethylene centrifuge tubes to gain the desired concentration composition. The effect of pH was studied by adjusting the initial pH of the experimental solutions between 2.0 and 7.0 with 0.01 mol L−1 HCl or NaOH solutions. The effect of NaCl concentration ranging from 0.001 to 0.2 M was investigated. The adsorption isotherms were measured for various concentrations of Cu2+ or Cd2+ at four different temperatures (293–323 K). The adsorption kinetics were studied by placing the PVA/GO nanofibers in 400 mL of Cu2+ or Cd2+ solution at 30 °C and testing the concentrations of Cu2+ or Cd2+ in solution at definite time intervals. The Cu2+ or Cd2+ concentrations were measured using atomic absorption spectroscopy (AAS). The values provided for the above experimental data were the average of three repeated measurements, and the relative errors were less than 5%. The adsorption capacity was calculated by the following equation:where Qe (mg g−1) is the adsorption capacity, Co (mg L−1) is the initial metal ion concentration, Ce (mg L−1) is the final metal ion concentration, Vm (L) is the volume of the metal ion solution, and M (g) is the mass of the adsorbent.
The above Cu2+ or Cd2+ adsorbed PVA/GO nanofibers were put into a 50 mL hydrochloric acid solution at pH 1 for desorption. After 2 h stirring at 20 °C, these nanofibers were rinsed with distilled water and dried in vacuum freezing drying oven. The regenerated PVA/GO nanofibers were then subjected to repeated adsorption/desorption cycles as described above to evaluate the recyclability of the PVA/GO nanofibers.
3. Results and discussion
3.1. Characterization
The morphology of GO and PVA/GO nanofibers are shown in Fig. 2. The SEM image shown in Fig. 2(a) and (b) reveal that the PVA/GO nanofibers without beads are randomly deposited to form a nonwoven mat. The cross sections of PVA/GO nanofibers are round and their surfaces are smooth. These results indicate that high quantity of PVA/GO nanofibers can be obtained by electrospinning. TEM was used to identify the existence of individual GO sheets. The TEM image shown in Fig. 2(c) indicates that GO nanosheets were successfully synthesized and fully exfoliated into individual sheets. Fig. 2(d) shows the TEM image of PVA/GO composite nanofibers, where it is clearly observed that GO nanosheets were successfully loaded onto the nanofibers. | ||
| Fig. 2 (a) Low magnitude SEM image of PVA/GO nanofibers. (b) High magnitude SEM images of PVA/GO nanofibers. (c) TEM image of GO. (d) TEM image of PVA/GO nanofibers. | ||
Fig. 3 shows the XRD spectra of PVA, GO and the PVA/GO nanofibers. The typical diffraction peak of GO is observed at approximately 2θ = 10.41°, and that of PVA appears at approximately 2θ = 19.6°. However, the XRD spectra of the PVA/GO nanofibers reveals only the diffraction peak originating from PVA, the diffraction peak of GO disappears. These results suggest that GO nanosheets were uniformly dispersed in the PVA matrix, and the crystalline structure of PVA was barely influenced by the addition of GO.32,36
The Raman spectra of PVA, GO and PVA/GO nanofibers are shown in Fig. 4. The spectrum of PVA shows a band at approximately 2908 cm−1. The Raman spectrum of GO shows two bands at approximately 1590 cm−1 and 1340 cm−1 which are assigned as D band and G band. However, the Raman spectrum of PVA/GO nanofiber displays only two peaks close to that of GO, the peak associated with PVA is not observed. This result clearly demonstrates that a large number of GO nanosheets are coated on the surface of the nanofiber.
The FTIR spectra of PVA, GO and PVA/GO nanofiber are shown in Fig. 5. The characteristic peaks of GO appear at 3440 cm−1, 1731 cm−1, 1401 cm−1, 1228 cm−1 and 1080 cm−1, corresponding to the stretching vibrations of O–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carboxyl and carbonyl), C–O (carboxyl), C–O (alkoxy), C–O (epoxy), respectively.37,38 This clearly confirms that various oxygen-containing groups exist on the basal planes and edges of GO. The spectrum of PVA exhibits a broad, strong band at approximately 3440 cm−1 relating hydroxyl groups. However, in the spectrum of PVA/GO nanofibers, this band broadens slightly and shifts to a lower wave number of 3292 cm−1. Additionally, the peak for carboxyl and carbonyl groups shifts from 1731 cm−1 in GO to a lower wave number (1718 cm−1) in PVA/GO nanofibers. This phenomenon may be caused by hydrogen bonding between the oxygen containing groups on surface of GO and the hydroxyl groups of PVA chains.32 In addition, the characteristic peak of PVA/GO nanofibers is in accordance with that of GO, which indicates that the PVA/GO nanofibers provide abundant oxygen-containing groups for heavy metal adsorption.
O (carboxyl and carbonyl), C–O (carboxyl), C–O (alkoxy), C–O (epoxy), respectively.37,38 This clearly confirms that various oxygen-containing groups exist on the basal planes and edges of GO. The spectrum of PVA exhibits a broad, strong band at approximately 3440 cm−1 relating hydroxyl groups. However, in the spectrum of PVA/GO nanofibers, this band broadens slightly and shifts to a lower wave number of 3292 cm−1. Additionally, the peak for carboxyl and carbonyl groups shifts from 1731 cm−1 in GO to a lower wave number (1718 cm−1) in PVA/GO nanofibers. This phenomenon may be caused by hydrogen bonding between the oxygen containing groups on surface of GO and the hydroxyl groups of PVA chains.32 In addition, the characteristic peak of PVA/GO nanofibers is in accordance with that of GO, which indicates that the PVA/GO nanofibers provide abundant oxygen-containing groups for heavy metal adsorption.
3.2. Effect of pH
The effects of pH on Cu2+ and Cd2+ adsorption are shown in Fig. 6. At a lower initial pH, small amounts of Cu2+ and Cd2+ could be adsorbed onto PVA/GO nanofibers. This phenomenon was mainly due to the competition between the high concentration of hydrogen ions and the metal ions for the adsorption sites on the surface of PVA/GO nanofibers. As the pH increased, the amount of adsorbed Cu2+ and Cd2+ was significantly greater. This may be due to the reduced number of hydrogen ions present in solution, which allowed more sites to form complexes with Cd2+ and Cu2+. However, a higher initial pH could also result in the precipitation of metal hydroxides. Thus, solubility product constants (Ksp) and initial Cu2+/Cd2+ concentrations were used to calculate the solution pH values at which the hydrolyzed species would not form. The Ksp used for Cu(OH)2 and Cd(OH)2 were 6.0 × 10−20 and 7.2 × 10−157, respectively. The calculated maximum pH values for Cu2+ or Cd2+ were 6.0 and 8.6, respectively. Therefore, Cu2+ began to form a precipitate at pH 6.0; whereas no observable precipitation of Cd2+ occurred. For the given pH values, all of the aqueous Cd2+ removed from solution were adsorbed onto PVA/GO nanofibers. To ensure that Cu2+ and Cd2+ ions were completely adsorbed, a pH of 5.8 ± 0.1 was selected for further investigations of Cu2+ and Cd2+ adsorption. | ||
| Fig. 6 Effect of pH on Cu2+ and Cd2+ adsorption onto PVA/GO nanofiber (C[Cu2+]initial = 30 mg L−1, C[Cd2+]initial = 40 mg L−1, m/V = 0.5 g/L, T = 303 K, I = 0.01 M NaCl). | ||
3.3. Effect of ionic strength
Effect of ionic strength on Cu2+ and Cd2+ sorption onto PVA/GO nanofibers is shown in Fig. 7. It is clear that a mild decrease in adsorption was observed with increasing ionic strength. This phenomenon can be attributed to the following: (1) as the NaCl concentration is decreasing, the formed electrical double layer complexes between Cu2+/Cd2+ and PVA/GO nanofibers are more beneficial to the adsorption of Cu2+/Cd2+ on PVA/GO nanofibers; (2) a large number of Na+ ions can compete with Cu2+/Cd2+ ions for binding sites on the surface of PVA/GO nanofibers, which reduce the adsorption of Cu2+/Cd2+ on PVA/GO nanofibers; (3) the activity coefficient of Cu2+ and Cd2+ in solution is influenced by NaCl concentrations, which is not beneficial to the transfer of the Cu2+/Cd2+ ions to the surface of PVA/GO nanofibers. Based on the above analysis, it can be inferred that the adsorptions of Cu2+ and Cd2+ on PVA/GO nanofibers are weakly dependent on the NaCl concentration. | ||
| Fig. 7 Effect of ionic strength on Cu2+ and Cd2+ adsorption onto PVA/GO nanofibers (C[Cu2+]initial = 30 mg L−1, C[Cd2+]initial = 40 mg L−1, m/V = 0.5 g/L, pH 5.8 ± 0.1, and T = 303 K). | ||
3.4. Effect of contact time and adsorption kinetics
The adsorption time is one of the important parameters that determines the adsorption equilibrium time of adsorbent in solid–liquid adsorption system.39 Fig. 8 shows the adsorption data of Cu2+ and Cd2+ onto PVA/GO nanofibers as a function of time. It can be seen that the Cu2+ and Cd2+ adsorption by PVA/GO nanofibers occurs rapidly within 10 min and then reaches equilibrium after 25 min. At the beginning 10 minutes, the fast adsorption may be due to the strong attraction between Cu2+ or Cd2+ and the adsorbent, leading to fast diffusion of the Cu2+/Cd2+ ions onto the surface of PVA/GO nanofibers. After 10 min, the gradually slow adsorption may be attributed to the reduction of binding sites on the surface of PVA/GO nanofibers and the weakening of interaction between Cu2+/Cd2+ ions and PVA/GO nanofibers. | ||
| Fig. 8 Effect of contact time on Cu2+ and Cd2+ adsorption onto PVA/GO nanofibers (C[Cu2+]initial = 30 mg L−1, C[Cd2+]initial = 40 mg L−1, m/V = 0.5 g/L, T = 303 K, pH 5.8 ± 0.1, I = 0.01 M NaCl). | ||
To further understand the observed adsorption phenomenon, the adsorption experimental data were fitted by the pseudo-first-order kinetic40 model and pseudo-second-order kinetic41 models. The pseudo-first-order equation is expressed in linear form:
log(qe − qt) = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t |
The pseudo-second-order equation is expressed in linear form:
The fitting plots of the above kinetic models for Cu2+/Cd2+ adsorption are shown in Fig. 9, and the kinetic parameters calculated from linear fitting equation are presented in Table 1. The R2 values of the pseudo-second-order model are greater than that of the pseudo-first-order model, indicating that the kinetics for Cu2+ and Cd2+ adsorption closely followed the pseudo-second-order model. Furthermore, the qe values (qe(cal)) calculated from the pseudo-second-order model for Cu2+ and Cd2+ adsorption are close to the experimental values (qe(exp)). The above results indicate that the adsorption rates of Cu2+ and Cd2+ ions on the surface of PVA/GO nanofibers are restricted by chemisorption.
 | ||
| Fig. 9 Pseudo-first-order (a) and pseudo-second-order (b) models for Cu2+ and Cd2+ adsorption onto PVA/GO nanofibers. | ||
| Metal ion | Pseudo-first-order | Pseudo second-order | |||||
|---|---|---|---|---|---|---|---|
| qe(cal) (mg g−1) | k1 (min−1) | R2 | qe(cal) (mg g−1) | k2 (g mg−1 min−1) | R2 | qe(exp) (mg g−1) | |
| Cu2+ | 16.73 | 0.06678 | 0.9705 | 23.1 | 0.00403 | 0.9961 | 21.2 |
| Cd2+ | 23.29 | 0.09442 | 0.9881 | 30.6 | 0.00863 | 0.9955 | 29.1 |
3.5. Adsorption isotherms
Adsorption isotherms are commonly employed to explore the adsorption mechanism. Here, the experimental equilibrium data at four different temperatures were fitted with Langmuir42 and Freundlich.43 The Langmuir isotherm is given in linear form by the following equation:where qe is the amount of the metal ions adsorbed per unit weight of adsorbent (mg g−1); qmax is the maximum adsorption capacity corresponding to a complete monolayer of coverage (mg g−1); ce is the equilibrium concentration of the metal ions (mg L−1); and kL is a constant related to the free energy of adsorption (L mg−1). The values of qmax and kL can be derived from the slope and intercept of ce/qe versus ce.
The Freundlich isotherm is linearly expressed as follows:
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = log qe = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kF + (1/n)log kF + (1/n)log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ce ce |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus log
qe versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ce.
ce.
The above model parameters evaluated from the plots are shown in Fig. 10, and presented in Table 2. The high R2 values reveal that the adsorption data of metal ions on PVA/GO nanofibers were well fitted by the Langmuir model, indicating that Cu2+ and Cd2+ adsorption on PVA/GO nanofibers are monolayer coverage. In addition, the maximum adsorption capacities calculated from the Langmuir model increased with increasing temperature, suggesting that the elevated temperature is beneficial to the adsorption of Cu2+ and Cd2+ on PVA/GO nanofibers. Langmuir constants (kL) indicate strong bonding of Cu2+ and Cd2+ onto PVA/GO nanofibers at the four different temperatures, and the Freundlich constants (1/n) at four different temperatures are smaller than 1, indicating the adsorption of Cu2+ and Cd2+ onto PVA/GO nanofibers are favorable processes.44,45 Furthermore, it is worth noting that the different adsorption capacity for the Cu2+ and Cd2+ of the PVA/GO nanofibers under the same temperature condition are observed. The reason can be due to the different affinities between the PVA/GO nanofibers and the Cu2+ and Cd2+ ions, which is probably caused by the different characteristic properties of Cu2+ and Cd2+, such as the ionic radius, electronegativity, polarizability and absolute hardness.
 | ||
| Fig. 10 Adsorption isotherms of Cu2+ (a and b) and Cd2+ (c and d) at different temperatures. (a) and (c) are Langmuir model simulations, and (b) and (d) are Freundlich model simulations. | ||
| Metal ions | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| qmax (mg g−1) | kL (L mg−1) | R2 | kF ((mg g−1)(L mg−1)1/n) | 1/n | R2 | ||
| Cu2+ | 293 K | 25.8 | 0.18752 | 0.9990 | 7.6481 | 0.2842 | 0.92114 |
| 303 K | 32.6 | 0.2001 | 0.9993 | 9.4258 | 0.3039 | 0.9401 | |
| 313 K | 39.4 | 0.2933 | 0.9985 | 15.2486 | 0.2333 | 0.9576 | |
| 323 K | 44.7 | 0.2978 | 0.9991 | 17.2973 | 0.2409 | 0.9223 | |
| Cd2+ | 293 K | 32.4 | 0.1154 | 0.9993 | 5.4736 | 0.4935 | 0.9263 |
| 303 K | 44.8 | 0.1382 | 0.9992 | 7.0718 | 0.4664 | 0.9447 | |
| 313 K | 53.1 | 0.1528 | 0.9971 | 11.7438 | 0.3781 | 0.9562 | |
| 323 K | 59.1 | 0.2272 | 0.9970 | 18.4680 | 0.3022 | 0.9634 | |
3.6. Adsorption thermodynamics
Thermodynamic parameters determine the degrees of spontaneity and feasibility inherent to an adsorption process. The thermodynamic parameters can be calculated from the isotherms at different temperature. The calculation equation is as follows:
ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 K0
| (1) |
The standard enthalpy change (ΔH0) and the standard entropy change (ΔS0) are calculated from the following equation:
Linear plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 versus 1/T for Cu2+/Cd2+ adsorption onto PVA/GO nanofibers are shown in Fig. 11. The values of ΔH0 and ΔS0 are obtained from the slopes and intercepts of the plots of ln
K0 versus 1/T for Cu2+/Cd2+ adsorption onto PVA/GO nanofibers are shown in Fig. 11. The values of ΔH0 and ΔS0 are obtained from the slopes and intercepts of the plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 versus 1/T. These parameters for Cu2+/Cd2+ onto PVA/GO nanofibers are summarized in Table 3. Negative values of ΔG0 indicate that the adsorptions of Cu2+ and Cd2+ onto PVA/GO nanofibers were spontaneous. The values of ΔG0 for Cu2+ and Cd2+ adsorption tend to be more negative with increasing temperatures, indicating that the higher temperature is helpful to the adsorption of the metal ions onto PVA/GO nanofibers. The positive values of ΔH0 indicate that the adsorption processes were endothermic.46,47 The positive values of ΔS0 indicate that randomness was increased during the adsorption process.48
K0 versus 1/T. These parameters for Cu2+/Cd2+ onto PVA/GO nanofibers are summarized in Table 3. Negative values of ΔG0 indicate that the adsorptions of Cu2+ and Cd2+ onto PVA/GO nanofibers were spontaneous. The values of ΔG0 for Cu2+ and Cd2+ adsorption tend to be more negative with increasing temperatures, indicating that the higher temperature is helpful to the adsorption of the metal ions onto PVA/GO nanofibers. The positive values of ΔH0 indicate that the adsorption processes were endothermic.46,47 The positive values of ΔS0 indicate that randomness was increased during the adsorption process.48
 | ||
Fig. 11 Linear plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K0 versus 1/T for Cu2+ (a) and Cd2+ (b) adsorption onto PVA/GO nanofibers at 293, 303, 313 and 323 K. K0 versus 1/T for Cu2+ (a) and Cd2+ (b) adsorption onto PVA/GO nanofibers at 293, 303, 313 and 323 K. | ||
| Metal ion | T (K) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) |
|---|---|---|---|---|
| Cu2+ | 293 | −19.2 | 20.3 | 140.2 |
| 303 | −20.5 | |||
| 313 | −21.9 | |||
| 323 | −23.2 | |||
| Cd2+ | 293 | −19.7 | 27.1 | 156.4 |
| 303 | −20.9 | |||
| 313 | −22.3 | |||
| 323 | −23.9 |
3.7. Regeneration study of PVA/GO nanofibers
The possibility of recycling the PVA/GO nanofibers was investigated by immersing the Cu2+-PVA/GO or Cd2+-PVA/GO nanofibers in hydrochloric acid (pH 1). The desorption efficiencie is shown in Fig. 12. It is observed that the first cycle resulted in 93.6% and 95.2% desorption for Cu2+ and Cd2+, respectively. The desorption efficiency for Cu2+ and Cd2+ reduced only after the 8th desorption to approximately 15.5% and 13.8%, respectively. The reduction in desorption efficiency may be due to the loss of adsorption sites after each cycle.49 Moreover, compared with the first cycle of regeneration, a 0.98% weight loss was observed after the 8th cycles of regeneration, indicating that the decrease of adsorption capacity can also be caused by the loss of the PVA/GO nanofibers themselves. The above results indicate that PVA/GO nanofibers can be used as an ideal heavy metal adsorbent and they have potential for use in several practical applications.3.8. Adsorption mechanism of Cu2+ and Cd2+ onto PVA/GO nanofibers
The adsorption of Cu2+ and Cd2+ onto PVA/GO nanofibers was confirmed by FT-IR and XPS analyses of PVA/GO-metal nanofiber samples. The FTIR spectra of PVA/GO, PVA/GO-Cu and PVA/GO-Cd nanofibers are shown in Fig. 13. The spectrum of PVA/GO nanofibers reveals vibration bands corresponding to O–H stretching at 3292 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching at 1718 cm−1, C
O stretching at 1718 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching at 1633 cm−1, C–O stretching at 1423 cm−1, C–O–C stretching at 1240 cm−1, C–O stretching at 1141 cm−1 and C–OH stretching at 1089 cm−1. The peaks at 1718 and 1423 cm−1 correspond to the C
C stretching at 1633 cm−1, C–O stretching at 1423 cm−1, C–O–C stretching at 1240 cm−1, C–O stretching at 1141 cm−1 and C–OH stretching at 1089 cm−1. The peaks at 1718 and 1423 cm−1 correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from carboxyl and carbonyl groups and C–O from carboxyl groups, respectively. These two peaks were shifted to 1698 and 1410 cm−1 for PVA/GO-Cu nanofibers and to 1685 and 1401 cm−1 for PVA/GO-Cd nanofibers, respectively. These changes are clear evidences of successful adsorption of metal ions onto PVA/GO nanofibers. It is worth noting that the peaks at 3929, 1240, 1141 and 1089 cm−1, which correspond to hydroxyl, alkoxy groups and epoxide, in all spectra did not change significantly after heavy metal ions adsorption. Thus, it can be concluded that both Cu2+ and Cd2+ bind primarily to the carboxyl and carbonyl groups in PVA/GO nanofibers. In addition to FT-IR, the adsorption of Cu2+ and Cd2+ on PVA/GO nanofibers was confirmed through XPS analysis, as shown in Fig. 14. In Fig. 14(a) and (b), the presence of Cu2+ in the PVA/GO-Cu nanofibers sample, at approximately 932.6 eV, confirms its successful adsorption. Similarly, the adsorption of Cd2+ is supported by the presence of the two peaks at 405.1 and 411.7 eV (Fig. 14(c) and (d)). Thus, XPS results once again testified that metal ions adsorption onto PVA/GO nanofibers.
O from carboxyl and carbonyl groups and C–O from carboxyl groups, respectively. These two peaks were shifted to 1698 and 1410 cm−1 for PVA/GO-Cu nanofibers and to 1685 and 1401 cm−1 for PVA/GO-Cd nanofibers, respectively. These changes are clear evidences of successful adsorption of metal ions onto PVA/GO nanofibers. It is worth noting that the peaks at 3929, 1240, 1141 and 1089 cm−1, which correspond to hydroxyl, alkoxy groups and epoxide, in all spectra did not change significantly after heavy metal ions adsorption. Thus, it can be concluded that both Cu2+ and Cd2+ bind primarily to the carboxyl and carbonyl groups in PVA/GO nanofibers. In addition to FT-IR, the adsorption of Cu2+ and Cd2+ on PVA/GO nanofibers was confirmed through XPS analysis, as shown in Fig. 14. In Fig. 14(a) and (b), the presence of Cu2+ in the PVA/GO-Cu nanofibers sample, at approximately 932.6 eV, confirms its successful adsorption. Similarly, the adsorption of Cd2+ is supported by the presence of the two peaks at 405.1 and 411.7 eV (Fig. 14(c) and (d)). Thus, XPS results once again testified that metal ions adsorption onto PVA/GO nanofibers.
 | ||
| Fig. 14 XPS of survey scan (a) and high-resolution scan (b) of PVA/GO nanofibers with adsorbed Cu2+, and survey scan (c) and high-resolution scan (d) of PVA/GO nanofibers with adsorbed Cd2+. | ||
4. Conclusions
Novel PVA/GO nanofibers were successfully prepared by electrospinning and employed as adsorbents for Cu2+ and Cd2+ removal. High content of GO can be uniformly dispersed in PVA to form PVA/GO nanofibers. The adsorption of Cu2+ and Cd2+ onto PVA/GO nanofibers increased with increasing pH, and only slightly decreased with the increase of ionic strength. The adsorption of Cu2+ and Cd2+ onto PVA/GO nanofibers is a rapid process that reached equilibrium within 25 min. The kinetic adsorption data followed the pseudo second-order kinetic model, and the equilibrium adsorption data can be well fitted with the Langmuir model. The thermodynamic parameters suggested that adsorption of Cu2+ and Cd2+ onto PVA/GO nanofibers were endothermic and spontaneous processes. The desorption efficiencies for Cu2+ and Cd2+ kept a high level and reduced only after the 8th adsorption/desorption cycle to approximately 15.5% and 13.8%, respectively. The FTIR and XPS results showed that the carboxyl and carbonyl groups on the surface of PVA/GO nanofibers are the main participants in the adsorption of Cu2+ and Cd2+. These results demonstrate that PVA/GO nanofibers could be efficient, nontoxic, regenerable and economical adsorbents due to their excellent reusability and outstanding performance in toxic heavy metals removal from wastewater.Acknowledgements
The authors gratefully acknowledge the financial support provided by the National Natural Science Fund of China (Foundation of Guangdong Province of China; No. U1401235).References
- T. Wang, W. Liu, L. Xiong, N. Xu and J. Ni, Chem. Eng. J., 2013, 215, 366–374 CrossRef.
- Y. Pang, G. Zeng, L. Tang, Y. Zhang, Y. Liu, X. Lei, Z. Li, J. Zhang and G. Xie, Desalination, 2011, 281, 278–284 CrossRef CAS.
- C. Zhijiang, J. Jianru, Z. Qing and Y. Haizheng, RSC Adv., 2015, 5, 82310–82323 RSC.
- L. Xu, X. Lu and X. Cheng, RSC Adv., 2015, 5, 79022–79030 RSC.
- B. Biškup and B. Subotić, Sep. Sci. Technol., 2005, 39, 925–940 CrossRef.
- M. M. Rao, A. Ramesh, G. P. C. Rao and K. Seshaiah, J. Hazard. Mater., 2006, 129, 123–129 CrossRef PubMed.
- L. V. Gurgel and L. F. Gil, Water Res., 2009, 43, 4479–4488 CrossRef CAS PubMed.
- X. Wang, J. Lu and B. Xing, Environ. Sci. Technol., 2008, 42, 3207–3212 CrossRef CAS PubMed.
- X. Deng, L. Lu, H. Li and F. Luo, J. Hazard. Mater., 2010, 183, 923–930 CrossRef CAS PubMed.
- J. Xu, L. Wang and Y. Zhu, Langmuir, 2012, 28, 8418–8425 CrossRef CAS PubMed.
- G. Zhao, J. Li, X. Ren, C. Chen and X. Wang, Environ. Sci. Technol., 2011, 45, 10454–10462 CrossRef CAS PubMed.
- S. T. Yang, Y. Chang, H. Wang, G. Liu, S. Chen, Y. Wang, Y. Liu and A. Cao, J. Colloid Interface Sci., 2010, 351, 122–127 CrossRef CAS PubMed.
- G. Zhao, X. Ren, X. Gao, X. Tan, J. Li, C. Chen, Y. Huang and X. Wang, Dalton Trans., 2011, 40, 10945–10952 RSC.
- R. Sitko, E. Turek, B. Zawisza, E. Malicka, E. Talik, J. Heimann, A. Gagor, B. Feist and R. Wrzalik, Dalton Trans., 2013, 42, 5682–5689 RSC.
- T. Yang, L.-H. Liu, J.-W. Liu, M.-L. Chen and J.-H. Wang, J. Mater. Chem., 2012, 22, 21909–21916 RSC.
- D. Li, J. T. McCann, Y. Xia and M. Marquez, J. Am. Ceram. Soc., 2006, 89, 1861–1869 CrossRef CAS.
- Z. Liu, D. D. Sun, P. Guo and J. O. Leckie, Nano Lett., 2007, 7, 1081–1085 CrossRef CAS PubMed.
- X. Lu, J. Zhou, Y. Zhao, Y. Qiu and J. Li, Chem. Mater., 2008, 20, 3420–3424 CrossRef CAS.
- Q. Bao, H. Zhang, J. x. Yang, S. Wang, D. Y. Tang, R. Jose, S. Ramakrishna, C. T. Lim and K. P. Loh, Adv. Funct. Mater., 2010, 20, 782–791 CrossRef CAS.
- L. Chu, S. Deng, R. Zhao, Z. Zhang, C. Li and X. Kang, RSC Adv., 2015, 5, 102625–102632 RSC.
- J. Miao, M. Miyauchi, T. J. Simmons, J. S. Dordick and R. J. Linhardt, J. Nanosci. Nanotechnol., 2010, 10, 5507–5519 CrossRef CAS PubMed.
- X. Lu, C. Wang and Y. Wei, Small, 2009, 5, 2349–2370 CrossRef CAS PubMed.
- D. Bjorge, N. Daels, S. De Vrieze, P. Dejans, T. Van Camp, W. Audenaert, J. Hogie, P. Westbroek, K. De Clerck and S. W. Van Hulle, Desalination, 2009, 249, 942–948 CrossRef CAS.
- K. Desai, K. Kit, J. Li, P. Michael Davidson, S. Zivanovic and H. Meyer, Polymer, 2009, 50, 3661–3669 CrossRef CAS.
- T. J. Sill and H. A. von Recum, Biomaterials, 2008, 29, 1989–2006 CrossRef CAS PubMed.
- E. S. Costa-Júnior, E. F. Barbosa-Stancioli, A. A. P. Mansur, W. L. Vasconcelos and H. S. Mansur, Carbohydr. Polym., 2009, 76, 472–481 CrossRef.
- Z. Ma, M. Kotaki, R. Inai and S. Ramakrishna, Tissue Eng., 2005, 11, 101–109 CrossRef CAS PubMed.
- K. T. Shalumon, N. S. Binulal, N. Selvamurugan, S. V. Nair, D. Menon, T. Furuike, H. Tamura and R. Jayakumar, Carbohydr. Polym., 2009, 77, 863–869 CrossRef CAS.
- C. S. Ki, E. H. Gang, I. C. Um and Y. H. Park, J. Membr. Sci., 2007, 302, 20–26 CrossRef CAS.
- S. Abbasizadeh, A. R. Keshtkar and M. A. Mousavian, Chem. Eng. J., 2013, 220, 161–171 CrossRef CAS.
- S. Wu, F. Li, Y. Wu, R. Xu and G. Li, Chem. Commun., 2010, 46, 1694–1696 RSC.
- J. Liang, Y. Huang, L. Zhang, Y. Wang, Y. Ma, T. Guo and Y. Chen, Adv. Funct. Mater., 2009, 19, 2297–2302 CrossRef CAS.
- C. Wang, Y. Li, G. Ding, X. Xie and M. Jiang, J. Appl. Polym. Sci., 2013, 127, 3026–3032 CrossRef CAS.
- S. Pavagadhi, A. L. L. Tang, M. Sathishkumar, K. P. Loh and R. Balasubramanian, Water Res., 2013, 47, 4621–4629 CrossRef CAS PubMed.
- P. Tan, J. Sun, Y. Hu, Z. Fang, Q. Bi, Y. Chen and J. Cheng, J. Hazard. Mater., 2015, 297, 251–260 CrossRef CAS PubMed.
- Y. Xu, W. Hong, H. Bai, C. Li and G. Shi, Carbon, 2009, 47, 3538–3543 CrossRef CAS.
- W. Song, X. Wang, Q. Wang, D. Shao and X. Wang, Phys. Chem. Chem. Phys., 2015, 17, 398–406 RSC.
- J. H. Chen, H. T. Xing, H. X. Guo, W. Weng, S. R. Hu, S. X. Li, Y. H. Huang, X. Sun and Z. B. Su, J. Mater. Chem. A, 2014, 2, 12561 CAS.
- J. Zhu, S. Wei, H. Gu, S. B. Rapole, Q. Wang, Z. Luo, N. Haldolaarachchige, D. P. Young and Z. Guo, Environ. Sci. Technol., 2011, 46, 977–985 CrossRef PubMed.
- S. Lagergren, Zur Theorie der sogenannten Absorption gelöster Stoffe, PA Norstedt & söner, 1898 Search PubMed.
- Y.-S. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- H. Freundlich, J. Phys. Chem., 1906, 57, 385–470 CAS.
- V. Chandra, J. Park, Y. Chun, J. W. Lee, I.-C. Hwang and K. S. Kim, ACS Nano, 2010, 4, 3979–3986 CrossRef CAS PubMed.
- X. Mi, G. Huang, W. Xie, W. Wang, Y. Liu and J. Gao, Carbon, 2012, 50, 4856–4864 CrossRef CAS.
- Y. Bulut and Z. Tez, J. Hazard. Mater., 2007, 149, 35–41 CrossRef CAS PubMed.
- C.-S. Zhu, L.-P. Wang and W.-B. Chen, J. Hazard. Mater., 2009, 168, 739–746 CrossRef CAS PubMed.
- Y. Bulut and Z. Baysal, J. Environ. Manage., 2006, 78, 107–113 CrossRef CAS PubMed.
- M. Aliabadi, M. Irani, J. Ismaeili, H. Piri and M. J. Parnian, Chem. Eng. J., 2013, 220, 237–243 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |