Optimum nanoscale design in ferrite based nanoparticles for magnetic particle hyperthermia†
S. Liébana-Viñasa,
K. Simeonidisb,
U. Wiedwalda,
Z.-A. Lia,
Zh. Maa,
E. Myrovalib,
A. Makridisb,
D. Sakellarib,
G. Vourliasb,
M. Spasovaa,
M. Farlea and
M. Angelakeris*b
aFaculty of Physics and Center for Nanointegration (CENIDE), University of Duisburg-Essen, 47057 Duisburg, Germany
bDepartment of Physics, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece. E-mail: agelaker@auth.gr; Fax: +30 2310998172; Tel: +30 2310998172
First published on 25th July 2016
Abstract
The study demonstrates the multiplex enhancement of the magnetic hyperthermia response in ferrites by nanoscale design and tuning without sparing the biocompatibility of iron-oxide. We propose core/shell nanoparticles with a 7–9 nm ferrite core, either magnetically soft MnFe2O4 or hard CoFe2O4, encapsulated by a 2–3 nm Fe3O4 shell providing a core/shell interface. In this case, the exchange interaction between core and shell dramatically affects the macroscopic magnetic behavior and, at the same time, a biocompatible shell prevents interactions of the toxic cores with their environment. The tunable, yet superior, magnetic hyperthermia response is proven by an increase of the specific loss power by a factor of 24 for CoFe2O4–Fe3O4 core/shell particles. This gain is directly connected with the magnetic coupling strength at the core/shell interface and opens the possibility of further optimization.
Introduction
The implementation of magnetic nanoparticles (MNPs) to the localized treatment of cancer appears as one of the most dynamic and promising fields of nanoparticle research.1–5 Early indications of the potential of MNPs to generate heat when subjected to an external high frequency AC magnetic field have been followed by an intense effort to improve efficiency and investigate ways for the applicability of this effect, namely Magnetic Particle Hyperthermia (MPH), in current cancer treatment schemes.6–8 In particular, diverse series of nanoparticles have been examined with respect to the constituent materials, the size and the shape.9–13 Another important aspect is the modelling of magnetic particle hyperthermia for predicting optimized experimental parameters from the choice of materials to the in vitro and in vivo heat transfer in tissues.14 The specific loss power (SLP) comprises all material and tissue parameters in a single quantity determining the heating efficiency of a specimen. Eventually, the ability to incorporate nanoparticles into biological environments with minimum side effects is crucial for the success of this methodology.15,16 Therefore, much effort has been devoted not only in engineering optimized nanoparticles but also in their bio-functionalization, in order to ensure selective targeting towards cancer cells and fate-control of MNPs after treatment.17–20According to their high heating efficiency, chemical stability and low cytotoxicity, iron oxide nanoparticles appear as the most prevalent class of versatile biomedical mediators in both research studies and commercial products.21–23 Single phase iron oxides (Fe3O4, γ-Fe2O3) are easily and inexpensively synthesized in a wide range of nanoparticle sizes and ensembles offering versatility in the acquired magnetic properties and interactions.24–31 However, the substitution of Fe by other ions (MFe2O4, M = Mn, Co, Cu, Ni, Zn, Ti, Mg) in the ferrite structure results in a significant improvement of the magnetic response exploitable for hyperthermia applications.32–37 For instance, the combination of magnetically soft (MnFe2O4) and hard (CoFe2O4) phases in core/shell formulations can multiply the heating efficiency (SLP) when compared to their single-phase counterparts.38–41 The reason for this gain is the tuning of the effective magnetic anisotropy via exchange-coupling at the interface between a hard and a soft magnetic phase.38,42,43 In addition, the core/shell architecture of ferrite nanoparticles also successfully overcomes the superparamagnetic size constraints and offers design options for the hysteresis losses leading to more effective heat generation.27,44,45 Nevertheless, the high dissolution rate in biological systems, which is accompanied by the leaching of toxic metal ions, still constrains the implementation of pure ferrite nanoparticles in bio-applications.46
Here, we demonstrate a route to more bio-friendly MNPs with a strongly improved heating efficiency (SLP enhancement by a factor of 24). The success of the approach is the tuning of the interface area and the relative volumes of the core and the shell as well as the magnetic anisotropy of both components. To improve biocompatibility of a non-iron based system, we have chosen magnetite completely coating spherical MnFe2O4 and CoFe2O4 nanoparticles, resulting at the same time in a magnetically hard/soft interface. The core/shell nanoparticles were synthesized in a two-step synthetic route to study the effect of interface coupling in detail and demonstrate the potential as efficient magnetic hyperthermia mediators.
The core/shell design of two different ferrite systems allows the tuning and matching of the coercive field and magnetization values to optimized hyperthermia conditions. As a result, hyperthermia heating experiments show a superior behavior of these core/shell ferrite systems when compared to their single-phase counterparts.
Experimental
Single phase MnFe2O4 (diameter 9 nm) and CoFe2O4 (diameter 7 nm) nanoparticles were initially prepared to serve as seeds for the formation of the core/shell architecture and as reference samples to evaluate the effect of magnetite coating. They were synthesized by coprecipitation of Mn2+ or Co2+ together with Fe3+ from the corresponding acetates (Mn(CH3COO)2·4H2O or Co(CH3COO)2·4H2O) and acetylacetonate (Fe(acac)3) salts. The preparation protocol was adapted from the literature.10 In short, 2 mmol of Mn(CH3COO)2 or Co(CH3COO)2 and 4 mmol of Fe(acac)3 were first dissolved in 30 mL of octadecane. 4.5 mL of oleic acid were added to the mixture for maintaining a comparable particle diameter for both samples. Under magnetic stirring and argon flow, the mixture was heated up to 300 °C and kept at this temperature for 20 min to completely evaporate moisture. Then, the temperature was set to 315–320 °C and the reaction proceeded under reflux conditions for 30 min. After cooling, the precipitate was washed several times with ethanol and separated by centrifugation. The obtained nanoparticles were finally dispersed and stored in n-hexane.Following a similar synthetic procedure, MnFe2O4 and CoFe2O4 nanoparticles were covered by a layer of Fe3O4 in a second step. Thus, 78 mg in powder form of the pre-synthesized ferrite (MnFe2O4 or CoFe2O4) were dispersed in 30 mL of octadecane with 2 mmol of Fe(CH3COO)2, 4 mmol of Fe(acac)3, 2.3 mL of oleic acid and 2.2 mL of oleylamine. After the evaporation of low-boiling point solvents from the reaction medium, the mixture was heated up to 320 °C and kept at this temperature for 30 min. The final product of the reaction was cooled down to room temperature and the precipitate was thoroughly washed with ethanol. The resulting core/shell nanoparticles were finally dispersed in n-hexane. A small portion of the final solutions was dried in air at T = 50 °C in order to obtain powder for further structural and magnetic characterization.
The structural characterization of the synthesized nanoparticles was performed by means of X-ray diffraction (XRD) with a Rigaku Ultima+ powder diffractometer using Cu-Kα radiation. Detailed microstructural and morphological properties of the particles were investigated by high resolution and scanning transmission electron microscopy (HRTEM and STEM), the latter combined with energy dispersive X-ray spectroscopy (EDXS) for chemical composition analysis. We used a Philips Tecnai F20 Supertwin microscope operated at 200 kV with a field emission gun.
The effect of the magnetite shell is studied by the changes of the magnetic properties before and after coating by hysteresis loops and zero-field cooled/field-cooled (ZFC/FC) experiments. The measurements were performed on powder samples using a Quantum Design MPMS SQUID magnetometer operating at a maximum field of μ0H = 5 T and temperature range of 10–300 K. Corresponding minor loops (±30 mT) were also taken after demagnetizing the samples in order to estimate the energy product experienced by the hysteresis loops for external fields applied during hyperthermia tests. All magnetization values were corrected to their net oxide content considering the amount of surfactant as determined from the weight losses during thermogravimetric analysis (TGA) using a Perkin-Elmer STA6000 instrument under nitrogen gas flow.
The heating efficiency of the nanoparticle dispersions was recorded in the RF region, under a higher (765 kHz) and a lower (210 kHz) AC magnetic field of 30 mT in a 4.5 kW commercial inductive apparatus and a 1.2 kW Ambrell Easyheat 0112, respectively. The temperature was monitored using a GaAs-based fiber optic probe immersed in a test tube containing 1 mL of dispersion. The specific loss power (SLP) was derived from the slope of the temperature versus time curve following a typical data routine to isolate magnetic-origin heating contributions.47,48 The procedure is described in brief in ESI.†
Results and discussion
The precipitation of metallic salts in organic solvents is initiated by the oversaturation caused by high temperature rather than any other chemical parameter. In the absence of any oxidizing or reducing agent in the reacting mixture, the metals preserve the same valence as in the dissolved acetate or acetylacetonate salts as shown in XPS analysis for the CoFe2O4 shown as Fig. S1 in ESI.†The structural and morphological investigations on the nanoparticles under study shown in Fig. 1 present low magnification TEM images of the samples, high-resolution TEM images and the corresponding Fourier transforms. For each sample, the MNP diameters were measured using at least 300 particles and the resulting size distributions were fitted by log-normal functions. In this way, we confirm an average particle diameter increase, after the magnetite growth, as indirect evidence of successful coating of the MnFe2O4 and CoFe2O4 presynthesized seeds. The mean particle diameters of the initially prepared MnFe2O4 and CoFe2O4 nanoparticles were found to be 8.7 nm and 7.2 nm, respectively (Fig. S2 in ESI†). After magnetite coating, the particle diameter of both core/shell samples increases up to around 12 nm, yielding a Fe3O4 shell thickness of 1.6 and 2.5 nm in Fe3O4@MnFe2O4 and Fe3O4@CoFe2O4 core/shell nanoparticles, respectively. Moreover, according to the bimodal size distributions of the Fe3O4@CoFe2O4 core/shell nanoparticles (Fig. S2†), the sample is composed of two particle species with different diameters. The two contributions arise from (a) nanoparticles with a larger diameter (12.2 nm), corresponding to CoFe2O4–Fe3O4 core/shell particles and (b) a fraction of smaller nanoparticles (7.2 nm), which are pure Fe3O4 nanoparticles as confirmed by EDX measurements presented in Fig. S3, ESI.† The formation of a small fraction of pure Fe3O4 nanoparticles may be ascribed to the second stage of the synthesis process, during which some of the iron ions present in the reaction medium did not deposit on the pre-existing seeds of CoFe2O4 particles, but instead formed new Fe3O4 nanoparticles.
The HRTEM and FFT images (Fig. 1) suggest single crystalline ferrite particles and an epitaxial growth of the Fe3O4 layer for the core/shell structures. The increased diameter of coated particles is consistent with the enhanced crystallite size observed in the XRD patterns of core/shell samples as compared to the single-phase counterparts, as shown in Fig. 2. More specifically, the mean crystal size estimated by the application of Scherrer's equation is increased from 7.5 for MnFe2O4 and 8.2 nm for CoFe2O4 to 12.0 nm for both core/shell samples.49 Although the XRD analysis does not provide adequate distinction between the different ferrites due to their structural resemblance, it outlines the high crystallinity of the nanoparticles since the typical peaks of the spinel structure, such as (220), (311), (400), (511) and (440), appear.50
 | ||
| Fig. 2 XRD diagrams of the studied samples in comparison to expected reflections from MnFe2O4 (#10-0319), CoFe2O4 (#03-0864) and Fe3O4 (#19-0629) from JCPDS-PDF database.50 | ||
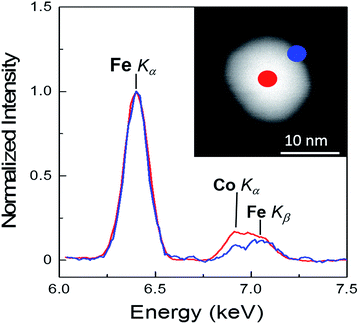
The elemental concentration of the different metallic ions in the samples has been determined by means of EDX spectroscopy in the TEM and reveals compositions very close to the stoichiometric values for the single-phase MnFe2O4 and CoFe2O4 ferrites. For the Fe3O4 coated nanoparticles the relative Co or Mn content decreases down to 13 ± 3%, as expected. Additionally, spatially resolved EDX spot and line scans were performed in different regions of individual core/shell nanoparticles in order to elucidate the elemental distribution within the nanoparticles' volume. As an example, such EDX measurements and analysis are shown in Fig. 3, applying high angle annular dark field (HAADF) scanning TEM (STEM) on the Fe3O4@CoFe2O4 core/shell nanoparticles. The EDX spectra recorded at the center (red) and on the edge (blue) are indicated in the HAADF image of the particle. Quantitative analysis of the EDX spectra containing Fe and Co is questionable here, since the energies of Fe Kβ and Co Kα partially overlap and the Co Kβ signal from a single particle is too small for precise evaluation. Nevertheless, the direct comparison of the two spectra normalized to the Fe Kα signal clearly reveals an enhanced Co-to-Fe ratio at the center and signal fitting gives an about 3-fold enhanced Co intensity as compared to the shell region. This finding has been confirmed on different particles, and thus the EDX point probes (Fig. 3) and the line scans (ESI Fig. S4†) unambiguously confirm the intended core/shell morphology.
Next, we turn to the static magnetic properties of the samples. Fig. 4 presents magnetic hysteresis loops of the Mn- and Co-ferrite samples before and after Fe3O4 coating at 10 and 300 K. Net magnetization values are corrected with respect to TGA curves shown in ESI Fig. S5.† The insets show the corresponding ZFC–FC curves acquired in the presence of an external magnetic field μ0H = 5 mT. MnFe2O4 ferrite nanoparticles show superparamagnetism at room temperature contrary to more anisotropic CoFe2O4 where ferromagnetism seems to dominate (as depicted by the approach to saturation and higher Ms values and the following Fig. 5) consistent with their small average diameters. For both samples, typical ferromagnetic responses at 10 K are evident, together with the increase of the coercive field with decreasing temperature. CoFe2O4 shows a higher magnetocrystalline anisotropy in the blocked state as compared to MnFe2O4, reaching a huge coercive field μ0HC ∼ 2 T at T = 10 K (Fig. 4b). Moreover, the expected superparamagnetic blocking temperatures, TB, agree well with the literature considering the nanoparticle volumes and magnetic anisotropies.26
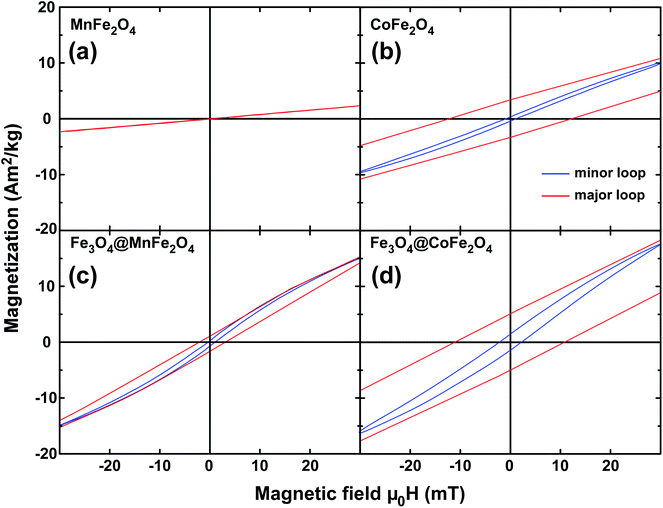 | ||
| Fig. 5 Minor hysteresis loops recorded at 30 mT for pure (a) and magnetite-coated MnFe2O4 (c) at 300 K. Corresponding loops of pure (b) and magnetite-coated (d) CoFe2O4 nanoparticles at T = 300 K. | ||
More interesting here is the change of the magnetic properties caused by the Fe3O4 coating of the ferrites. The hysteresis loops of the core/shell nanoparticles show an increase of the saturation magnetization and decrease of the coercive field for both systems. These features are consistent with the coexistence of two magnetic phases, one magnetically hard and one soft species, where ultimate saturation magnetization and coercive field values are expected to be the weighted average of the corresponding values of the two components. Besides, the decrease of the coercive field is an indication for exchange coupling in a composite material which consists of hard and soft magnets, commonly referred to as exchange-spring magnets.51
For MnFe2O4, the effect of the Fe3O4 shell in the magnetic response of the sample is mild due to the soft magnetic character exhibited by both phases sharing a nanoscale interface. More specifically, MnFe2O4 nanoparticles initially show a superparamagnetic blocking near 80 ± 10 K, while for the corresponding core/shell particles the major portion of particles shifts at about 250 ± 20 K. However, at ambient temperature, we obtain a non-zero coercive field and an increase of the magnetization in the presence of the Fe3O4 layer. We expect a stronger influence in the magnetic response in the core/shell sample with a CoFe2O4 core, a typical hard magnetic material when replacing a typical soft magnetic material core such as MnFe2O4.41
The corresponding interaction of the core and shell materials results in an increase of the saturation magnetization to around 67 A m2 kg−1 while the coercive field μ0H is kept at a level of 10 mT at room temperature, assuring ferromagnetic features, and thus matching the optimized conditions for hyperthermia (higher hysteresis losses). At low temperatures (blocked state) the coercive field increases to about μ0H = 1 T (Fig. 4d), which is nearly half of the value of the pure CoFe2O4 particles. Moreover, the magnetic blocking temperatures TB shift significantly towards higher temperatures reflecting the larger diameters while the (average) magnetic anisotropies decrease due to Fe3O4 coating. The mean blocking temperature of single CoFe2O4 nanoparticles is around 230 ± 20 K but overcomes ambient temperature in the core/shell sample. An overview of magnetic features exists as Table 1 in ESI.†
To further unravel the role of magnetic features on hyperthermia efficiency, we recorded minor hysteresis loops (±30 mT at ambient temperature) delivering the hysteresis losses per magnetic cycle. Note that these losses per cycle were determined for powder samples at quasi-static conditions, and are thus expected to increase under AC conditions. Fig. 5 presents both the major and minor loops at T = 300 K. For superparamagnetic MnFe2O4 nanoparticles heating via hysteresis losses is absent (Fig. 5a) while the coating by Fe3O4 results in a mild hysteresis occurrence at 300 K and the corresponding loss per cycle is calculated to be 0.25 J kg−1. More striking is the enhancement of Fe3O4@CoFe2O4 nanoparticles arising from the much harder CoFe2O4 seeds. The initial value of 0.20 J kg−1 in single-phase nanoparticles reaches 1.10 J kg−1 in the core/shell sample.
Finally, the heat losses of the particles in dispersion under AC magnetic fields, i.e. the magnetic particle hyperthermia at an amplitude of 30 mT and frequencies of 210 kHz and 765 kHz, were recorded. In Fig. 6, we present the SLP values for MnFe2O4 and CoFe2O4 nanoparticles and the corresponding core/shell architectures. The SLP calculation is based on a rigorous methodology already published,47,48 giving special care to discriminate magnetic origin heating from non-magnetic contributions. In ESI† a brief overview of this procedure is given, together with the initial hyperthermia curves (Fig. S6-ESI†). In Fig. 6, estimations of the hysteresis losses arising from the Fig. 5 DC minor loops are given (right scale) as a reference efficiency threshold. For MnFe2O4 we observe a low specific loss power hardly reaching 20 W g−1 at 765 kHz. This is a further indication of the weak contribution of hysteresis losses during the application of the AC magnetic field, as expected for softer magnetic materials. More specifically, the non-zero SLP in the case of superparamagnetic MnFe2O4 nanoparticles suggests that Néel relaxation remains the dominant heating mechanism. However, the coating of nanoparticles by the Fe3O4 layer and the subsequent activation of a magnetic hysteresis loss pathway for heat dissipation results in SLP enhancement by a factor of two in Fe3O4@MnFe2O4 nanoparticles. A more pronounced effect appears in Fe3O4@CoFe2O4 nanoparticles. In such a case an absolute value of around 450 W g−1 at 765 kHz and 30 mT is thus a factor of 24 larger as compared to the single-phase CoFe2O4 reference. The considerable SLP enhancement at 765 kHz with respect to the one at 210 kHz is a natural outcome if we consider that, within this frequency range, the driving force of magnetic heating efficiency, “χ” (the out-of-phase (imaginary) component of susceptibility), can be approximated as frequency independent, and thus the SLP parameter is expected to increase linearly with frequency.52,53 Importantly, our experimental SLP results are consistent with the DC minor loop hysteresis losses (Fig. 6, right scale) and reflect once again a more efficient heating mechanism via hysteresis losses as a consequence of the pronounced magnetic features shown in Fig. 4d and 5d, as dictated by the Fe(soft)/Co(hard) interface.
In summary, our results suggest that coating of ferrite nanoparticles by magnetite shells potentially provides multifold advantages desirable for the exploitation in magnetic hyperthermia applications. As previously reported for the combination of MnFe2O4 and CoFe2O4 core/shell particles,40 this geometry with materials having different magnetic anisotropies and magnetization triggers exchange-spring effects at their core/shell interface and therefore, significantly enhances hysteresis losses during AC field treatment. The success of the approach is the matching of the absolute and relative dimensions of the core diameter and the shell thickness as well as the anisotropy and magnetization of each of the components in use. In the present case, both the seed nanoparticle diameter (7–9 nm) and the magnetite shell thickness (1.5–2.5 nm) do lie in the optimum range for the maximum positive-contributing coupling. The initially higher anisotropy of the CoFe2O4 nanoparticles is responsible for the remarkable increase of the SLP in the Fe3O4@CoFe2O4 core/shell morphology. In this specific case, the design and matching of the volumes and magnetic anisotropies of the core and the shell, in conjunction with their high total magnetization and the interface exchange, results in the optimized hysteretic losses under the applied hyperthermia conditions.54,55 Such a matching of the MNP size, shape, and anisotropy to the experimental conditions (AC frequency and field amplitude) results in a rise in SLP trends by a factor of 5.5 for 15 nm CoFe2O4–MnFe2O4 core/shell MNPs38 and of 14 for 60 nm CoFe2O4–Zn0.4Fe2.6O4 core/shell MNPs.44 An interesting question arises from the extraordinarily high absolute SLP magnitudes in these works even for the single-phase ferrites.32 It seems that the absolute value of the SLP magnitude is much more sensitive not only to mixing and matching shell thicknesses and core diameters but also to AC field parameters. On the other hand, a recent theoretical study failed to predict such high values, following a core/shell model,56 in excellent agreement with our results and relevant studies.57,58
Eventually, the presence of the Fe3O4 shell renders these species much safer for biomedical applications and introduces more design possibilities for the safe use of Mn and Co ferrites in biological environments. Although direct cytotoxicity of MnFe2O4 and CoFe2O4 nanoparticles is usually low in reasonable concentrations, the secondary toxicity caused by released Mn2+ and Co2+ ions should also be considered. Once degradation of the particles sets in, these cations appear as extremely toxic on cells even at very low concentrations due to their high mobility compared to that of the nanoparticles. On the contrary, Fe ions are completely safe in a wide range of concentrations.59 Therefore, magnetite shells may act as protective layers inhibiting the dissolution of toxic ions to the outer environment during magnetic hyperthermia treatment. In a next step, the present core/shell nanoparticles have to be grown60 in or transferred to aqueous solution61 allowing direct in vitro and in vivo sustainability and toxicity testing.
Conclusions
Magnetic nanoparticles consisting of a ferrite core either magnetically soft, such as MnFe2O4, or hard, such as CoFe2O epitaxially coated by a magnetite shell were synthesized following a two-stage wet chemistry procedure. The core/shell architecture was selected as a pathway to incorporate materials beyond typical iron oxides as magnetic hyperthermia mediators. In this design, the magnetite shell is coupled to the ferrite core and forms an exchange-spring magnet on the nanoscale while a promising biocompatibility impact is primarily guaranteed. Thus, a novel mediator such as Co-ferrite may be incorporated as a core coated by Fe3O4, resulting in a strongly enhanced magnetic hyperthermia efficiency, up to a factor of 24. This beneficial outcome is the result of the precise tuning of the core/shell geometry resulting both with the magnetic anisotropy matching the optimum hyperthermia conditions and the magnetic hysteresis losses becoming the dominant heating pathway.Acknowledgements
Financial support by the IKYDA2013 bilateral Greek-German collaboration scheme is acknowledged. Financial support from the European Community's Seventh Framework Program (FP7-NMP) under grant agreement no. 280670 (REFREEPERMAG) and the University of Duisburg-Essen is acknowledged.Notes and references
- S. Dutz and R. Hergt, Nanotechnology, 2014, 25, 452001 CrossRef PubMed.
- C. S. S. R. Kumar and F. Mohammad, Adv. Drug Delivery Rev., 2011, 63, 789 CrossRef CAS PubMed.
- E. A. Périgo, G. Hemery, O. Sandre, D. Ortega, E. Garaio, F. Plazaola and F. J. Teran, Appl. Phys. Rev., 2015, 2, 041302 Search PubMed.
- S. Leulmi, X. Chauchet, M. Morcrette, G. Ortiz, H. Joisten, P. Sabon, T. Livache, Y. Hou, M. Carrière, S. Lequien and B. Dieny, Nanoscale, 2015, 7, 15904 RSC.
- D. Shi, M. E. Sadat, A. W. Dunn and D. B. Mast, Nanoscale, 2015, 7, 8209 RSC.
- Y. Qu, J. Li, J. Ren, J. Leng, C. Lin and D. L. Shi, Nanoscale, 2014, 6, 12408 RSC.
- T. Sadhukha, L. Niu, T. S. Wiedmann and J. Panyam, Mol. Pharmaceutics, 2013, 10, 1432 CrossRef CAS PubMed.
- A. C. Anselmo and S. Mitragotri, AAPS J., 2015, 17, 1041 CrossRef CAS PubMed.
- H. Mamiya, J. Nanomater., 2013, 2013, 752973 Search PubMed.
- Y.-J. Kim, Adv. Funct. Mater., 2013, 23, 5753 CrossRef CAS.
- W.-S. Lin, H.-M. Lin, H.-H. Chen, Y.-K. Hwu and Y.-J. Chiou, J. Nanomater., 2013, 28, 237439 Search PubMed.
- C. Pereira, A. M. Pereira, C. Fernandes, M. Rocha, R. Mendes, M. P. Fernández-García, A. Guedes, P. B. Tavares, J.-M. Grenèche, J. P. Araujo and C. Freire, Chem. Mater., 2012, 24, 1496 CrossRef CAS.
- C. Munoz-Menendez, I. Conde-Leboran, D. Baldomir, O. Chubykalo-Fesenko and D. Serantes, Phys. Chem. Chem. Phys., 2015, 17, 27812 RSC.
- L.-Y. Zhao, J.-Y. Liu, W.-W. Ouyang, D.-Y. Li, L. Li, L.-Y. Li and J.-T. Tang, Chin. Phys. B, 2013, 22, 108104 CrossRef.
- R. Di Corato, A. Espinosa, L. Lartigue, M. Tharaud, S. Chat, T. Pellegrino, C. Ménager, F. Gazeau and C. Wilhelm, Biomaterials, 2014, 35, 6400 CrossRef CAS PubMed.
- I. S. Smolkov, N. E. Kazantseva, K. N. Makoveckaya, P. Smolka, P. Saha and A. M. Granov, Mater. Sci. Eng., C, 2015, 48, 632 CrossRef PubMed.
- E.-K. Lim, T. Kim, S. Paik, S. Haam, Y.-M. Huh and K. Lee, Chem. Rev., 2015, 115, 327 CrossRef CAS PubMed.
- Y. Bao and K. M. Krishnan, J. Magn. Magn. Mater., 2005, 293, 15 CrossRef CAS.
- P. I. P. Soares, F. Lochte, C. Echevarria, L. C. J. Pereira, J. T. Coutinho, I. M. M. Ferreira, C. M. M. Novo and J. P. M. R. Borges, J. Nanotechnol., 2015, 26, 425704 CrossRef PubMed.
- D. Zhao, W. Huang, M. N. Rahaman, D. E. Day, D. Wang and Y. Gu, Mater. Sci. Eng., C, 2012, 32, 276 CrossRef CAS.
- A. Figuerola, R. Di Corato, L. Manna and T. Pellegrino, Pharmacol. Res., 2010, 62, 126 CrossRef CAS PubMed.
- A. Meffre, B. Mehdaoui, V. Kelsen, P. F. Fazzini, J. Carrey, S. Lachaize, M. Respaud and B. Chaudret, Nano Lett., 2012, 12, 4722 CrossRef CAS PubMed.
- I. Castellanos-Rubio, M. Insausti, E. Garaio, I. Gil de Muro, F. Plazaola, T. Rojoa and L. Lezama, Nanoscale, 2014, 6, 7542 RSC.
- S. Veintemillas-Verdaguer, M. Marciello, M. d. P. Morales, C. J. Serna and M. Andrés-Vergés, Prog. Cryst. Growth Charact. Mater., 2014, 60, 80 CrossRef CAS.
- D. Serantes, K. Simeonidis, M. Angelakeris, O. Chubykalo-Fesenko, M. Marciello, M. del Puerto Morales, D. Baldomir and C. Martinez-Boubeta, J. Phys. Chem. C, 2014, 118, 5927 CAS.
- P. Hugounenq, M. Levy, D. Alloyeau, L. Lartigue, E. Dubois, V. Cabuil, C. Ricolleau, S. Roux, C. Wilhelm, F. Gazeau and R. Bazzi, J. Phys. Chem. C, 2012, 116, 15702 CAS.
- C. Blanco-Andujar, D. Ortega, P. Southern, Q. A. Pankhurst and N. T. K. Thanh, Nanoscale, 2015, 7, 1768 RSC.
- S. Dutz, M. Kettering, I. Hilger, R. Müller and M. Zeisberger, Nanotechnology, 2011, 22, 265102 CrossRef PubMed.
- M. E. Sadat, R. Patel, J. Sookoor, S. L. Bud'ko, R. C. Ewing, J. Zhang, H. Xug, Y. Wang, G. M. Pauletti, D. B. Mast and D. Shi, Mater. Sci. Eng., C, 2014, 42, 52 CrossRef CAS PubMed.
- S. Nappini, E. Magnano, F. Bondino, I. Píš, A. Barla, E. Fantechi, F. Pineider, C. Sangregorio, L. Vaccari, L. Venturelli and P. Baglion, J. Phys. Chem. C, 2015, 119(45), 25529 CAS.
- D. Sakellari, K. Brintakis, A. Kostopoulou, E. Myrovali, K. Simeonidis, A. Lappas and M. Angelakeris, Mater. Sci. Eng., C, 2016, 58, 187 CrossRef CAS PubMed.
- I. Sharifi, H. Shokrollahi and S. Amiri, J. Magn. Magn. Mater., 2012, 324, 903 CrossRef CAS.
- M. Lin, J. Huang and M. Sha, J. Nanosci. Nanotechnol., 2014, 14, 792 CrossRef CAS PubMed.
- E. L. Verde, G. T. Landi, M. S. Carrião, A. L. Drummond, J. A. Gomes, E. D. Vieira, M. H. Sousa and A. F. Bakuzis, AIP Adv., 2012, 2, 032120 CrossRef.
- H. Das, N. Sakamoto, H. Aono, K. Shinozaki, H. Suzuki and N. Wakiya, J. Magn. Magn. Mater., 2015, 392, 91 CrossRef CAS.
- A. B. Raghvendra, D. T. Nanasaheb, K. C. Akhilesh and H. P. Shivaji, RSC Adv., 2015, 5, 47225 RSC.
- V. Gavrilov-Isaac, S. Neveu, V. Dupuis, D. Taverna, A. Gloter and V. Cabuil, Small, 2015, 11, 2614 CrossRef CAS PubMed.
- J.-H. Lee, J.-T. Jang, J.-S. Choi, S. H. Moon, S.-H. Noh, J.-W. Kim, J.-G. Kim, I.-S. Kim, K. I. Park and J. Cheon, Nat. Nanotechnol., 2011, 6, 418 CrossRef CAS PubMed.
- R. Cabreira-Gomes, F. G. Silva, R. Aquino, P. Bonville, F. A. Tourinho, R. Perzynski and J. Depeyrot, J. Magn. Magn. Mater., 2014, 368, 409 CrossRef CAS.
- M. Angelakeris, Z.-A. Li, M. Hilgendorff, K. Simeonidis, D. Sakellari, M. Filippousi, H. Tian, G. Van Tendeloo, M. Spasova, M. Acet and M. Farle, J. Magn. Magn. Mater., 2015, 381, 179 CrossRef CAS.
- Q. Song and Z. J. Zhang, J. Am. Chem. Soc., 2012, 134, 10182 CrossRef CAS PubMed.
- T. J. Daou, J. M. Grenèche, G. Pourroy, S. Buathong, A. Derory, C. Ulhaq-Bouillet, B. Donnio, D. Guillon and S. Begin-Colin, Chem. Mater., 2008, 20, 5869 CrossRef CAS.
- S. Liebana-Viñas, U. Wiedwald, A. Elsukova, J. Perl, B. Zingsem, A. S. Semisalova, V. Salgueiriño, M. Spasova and M. Farle, Chem. Mater., 2015, 27, 4015 CrossRef.
- S. H. Noh, W. Na, J. T. Jang, J. H. Lee, E. J. Lee, S. H. Moon, Y. Lim, J.-S. Shin and J. Cheon, Nano Lett., 2012, 12, 3716 CrossRef CAS PubMed.
- S. Liebana-Viñas, K. Simeonidis, Z.-A. Li, Z. Ma, E. Myrovali, A. Makridis, D. Sakellari, M. Angelakeris, U. Wiedwald, M. Spasova and M. Farle, J. Magn. Magn. Mater., 2016, 415, 20–23 CrossRef.
- S. J. Soenen, W. J. Parak, J. Rejman and B. Manshian, Chem. Rev., 2015, 115, 2109 CrossRef CAS PubMed.
- A. Chalkidou, K. Simeonidis, M. Angelakeris, T. Samaras, C. Martinez-Boubeta, L. Balcells, K. Papazisis, C. Dendrinou-Samara and O. Kalogirou, J. Magn. Magn. Mater., 2011, 323, 775 CrossRef CAS.
- K. Simeonidis, C. Martinez-Boubeta, L. Balcells, C. Monty, G. Stavropoulos, M. Mitrakas, A. Matsakidou, G. Vourlias and M. Angelakeris, J. Appl. Phys., 2013, 114, 103904 CrossRef.
- A. L. Patterson, Phys. Rev., 1939, 56, 978 CrossRef CAS.
- Joint Center for Powder Diffraction Studies, Powder Diffraction File, International Centre for Diffraction Data, Newtown Square, PA, 2004 Search PubMed.
- J. M. Soares, V. B. Galdino and F. L. A. Machado, J. Magn. Magn. Mater., 2014, 350, 69 CrossRef CAS.
- M. Kallumadil, M. Tadac, T. Nakagawa, M. Abe, P. Southern and Q. A. Pankhurst, J. Magn. Magn. Mater., 2009, 321, 1509 CrossRef CAS.
- H. Mamiya and B. Jeyadevan, Sci. Rep., 2011, 1, 157 Search PubMed.
- K. D. Bakoglidis, K. Simeonidis, D. Sakellari, G. Stefanou and M. Angelakeris, IEEE Trans. Magn., 2012, 48, 1320 CrossRef CAS.
- C. Martinez-Boubeta, K. Simeonidis, D. Serantes, I. Conde-Leborán, I. Kazakis, G. Stefanou, L. Peňa, R. Galceran, Ll. Balcells, C. Monty, D. Baldomir, M. Mitrakas and M. Angelakeris, Adv. Funct. Mater., 2012, 17, 3737 CrossRef.
- M. S. Carrião and A. F. Bakuzis, Nanoscale, 2016, 8, 8363–8377 RSC.
- Q. Zhang, I. Castellanos-Rubio, R. Munshi, I. Orue, B. Pelaz, K. Ines Gries, W. J. Parak, P. del Pino and A. Pralle, Chem. Mater., 2015, 27(21), 7380–7387 CrossRef CAS.
- Z. Nemati, J. Alonso, H. Khurshid, M. H. Phana and H. Srikanth, RSC Adv., 2016, 6, 38697–38702 RSC.
- H. Markides, M. Rotherham and A. J. El Haj, J. Nanomater., 2012, 2012, 614094 CrossRef.
- K. Simeonidis, S. Liébana-Viñas, U. Wiedwald, Z. Ma, Z.-A. Li, M. Spasova, O. Patsia, E. Myrovali, A. Makridis, D. Sakellari, I. Tsiaoussis, G. Vourlias, M. Farle and M. Angelakeris, RSC Adv., 2016, 6, 53107–53117 RSC.
- K. Giannousi, M. Menelaou, J. Arvanitidis, M. Angelakeris, A. Pantazaki and C. Dendrinou-Samara, J. Mater. Chem. B, 2015, 3, 5341–5351 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17892h |
| This journal is © The Royal Society of Chemistry 2016 |