A rhodamine-based sensor for Hg2+ and resultant complex as a fluorescence sensor for I−†
Abstract
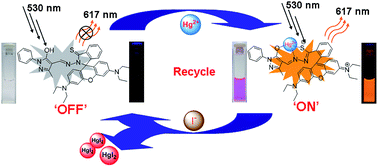
The recognition and sensing of ions have attracted growing attention because of the important role played by ions in biological, industrial and environmental processes. However, many sensors can only detect a single ion. We report here a rhodamine-based sensor with functions of dual ion detection. It displays a quick colorimetric and fluorometric sensing ability on 1 : 1 binding to Hg2+, with a change of color of solution (colorless to pink) and fluorescence color (dark to orange) due to Hg2+-induced opening of the spirolactam ring in the rhodamine structure. The detection limits were found to be 32 nM and 44 nM by absorption and fluorescence methods, respectively. More importantly, the resulting complex 1–Hg2+ can be used as a reversible fluorescence sensor for I−. With the addition of I−, the fluorescence intensity was quenched because Hg2+ in the complex was grabbed by I− attributed to the stronger binding force between Hg2+ and I−. Based on the “OFF–ON–OFF” behavior, a molecular device with “INHIBIT” logic gate function was constructed.

 Please wait while we load your content...
Please wait while we load your content...