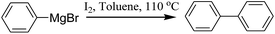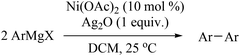Homocoupling of heteroaryl/aryl/alkyl Grignard reagents: I2-promoted, or Ni- or Pd- or Cu- or nano-Fe-based salt catalyzed†
Xing Li*,
Dongjun Li,
Yingjun Li,
Honghong Chang,
Wenchao Gao and
Wenlong Wei*
Department of Chemistry and Chemical Engineering, Taiyuan University of Technology, 79 West Yingze Street, Taiyuan 030024, People's Republic of China. E-mail: lixing@tyut.edu.cn
First published on 8th September 2016
Abstract
Five efficient processes for the homo-coupling of various Grignard reagents including aryl, heteroaryl and aliphatic ones in the presence of I2, Pd(OAc)2, Ni(OAc)2, CuI, and nano-Fe3O4 were developed, respectively.
The symmetrical biaryl unit is found in a number of natural products, pharmaceuticals, optical materials and functional molecules.1 Oxidative homo-coupling reactions of Grignard reagents have gained great attention as they provide an easy and efficient access for the construction of such compounds. Several kinds of transition metal promoters including cobalt salts,2 TlBr,3 TiCl4,4 vanadium salts,5 copper salts,6 etc.7 have been used in the presence of a reoxidant to carry out the oxidative coupling process in most cases.8 In these reactions, however, a stoichiometric or excess amount of transition metal is used for smooth transformation. Consequently, with the urgent demand for green chemistry process and sustainable development, the exploration of the suitable catalytic systems with efficient catalysts and inexpensive oxidants obviously becomes one of the most important topics. On the other hand, some common and easily available transition metal catalysts have been successfully used in the cross-coupling reactions of Grignard reagents with organic electrophiles and possessed the synthetic advantages such as high selectivity, broad substrate scopes and mild reaction conditions in recent years.9 In contrast, the catalyzed routes to symmetrical biaryls from Grignard reagents about them have not received as much attention although there have been several examples carried out on manganese-,10 iron-,11 cobalt-,12 ruthenium-13 and copper-catalyzed14 homo-coupling reactions. Therefore, we anticipate that similar Pd- and Ni-catalyzed methods can also be realized under certain conditions, and to the best of our knowledge, there have been no reports on I2-mediated, nano-Fe3O4- and CuI-catalyzed oxidative homo-coupling so far. Herein, we will report Pd(OAc)2-, Ni(OAc)2-, CuI-, and nano-Fe3O4-catalyzed, and I2-promoted routes to symmetrical biaryls from Grignard reagents.
First, when phenylmagnesium bromide was treated with 0.2 equiv. of I2 in toluene at 110 °C, the desired product was obtained in 37% yield (Table 1, entry 1). The yield was improved greatly by increasing the amount of I2 and the highest yield was provided when 0.8 equiv. I2 was used (Table 1, entry 4 vs. 2, 3 and 5).
With the promising results, the scope of Grignard reagents was next investigated and the results are shown in Table 2. The results indicated that a variety of Grignard reagents could also be quickly transformed into the corresponding products in the presence of I2. Grignard reagents having electron-poor or -rich groups on benzene ring underwent smoothly transformation to give products in good to high yields (Table 2, entries 1–7). The benzylmagnesium bromide could afford the corresponding product in 54% yield, although a longer time and higher temperature was required (Table 2, entry 8). Heteroaryl Grignard reagents such as pyridylmagnesium bromide and thienylmagnesium bromide were found be suitable substrates to give the homo-coupled products in good yields (Table 2, entries 9–11). Homo-couplings of arylmagnesium chloride also proceeded well under similar conditions (Table 2, entries 12–14). Excitedly, 1,4-diphenylbuta-1,3-diyne was provided in 62% yield when (phenylethynyl)magnesium bromide was used as a substrate (Table 2, entry 15). However, no product was detected for styrylmagnesium bromide (Table 2, entry 16).
| Entry | Grignard reagent | Product | t (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: Grignard reagent (0.3 mmol), I2 (0.8 equiv.), toluene (1.5 mL), temperature (110 °C).b Isolated yield.c 140 °C was used.d N.D. = not detected. | ||||
| 1 |  |
 |
48 | 96 |
| 2 |  |
 |
50 | 83 |
| 3 |  |
 |
48 | 88 |
| 4 |  |
 |
48 | 92 |
| 5 |  |
 |
48 | 86 |
| 6 |  |
 |
50 | 84 |
| 7 |  |
 |
50 | 91 |
| 8 | 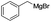 |
 |
48 | 54c |
| 9 |  |
 |
48 | 82 |
| 10 |  |
 |
48 | 76 |
| 11 |  |
 |
48 | 81 |
| 12 |  |
 |
48 | 89 |
| 13 |  |
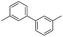 |
50 | 82 |
| 14 |  |
 |
48 | 87 |
| 15 |  |
 |
48 | 62 |
| 16 |  |
 |
48 | N.D.d |
Subsequently, our studies focused on the Pd-catalyzed homo-coupling of various types of Grignard reagents. It was found that through the use of Pd(OAc)2,15 a wide range of Grignard reagents, including arylmagnesium bromide, heteroarylmagnesium bromide, and arylmagnesium chloride could be effectively transformed in good to excellent yields in the presence of LiClO4 (Table 3, entries 1–7 and 9–14).16 Finally, benzylmagnesium bromide also represented a compatible substrate under the reflux conditions (Table 3, entry 8).
| Entry | Grignard reagent | Product | t (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: Grignard reagent (0.3 mmol), Pd(OAc)2 (10 mol%), LiClO4 (0.3 mmol, 1.0 equiv.), toluene (2.0 mL), temperature (100 °C).b Isolated yield.c 120 °C was used. | ||||
| 1 |  |
 |
15 | 98 |
| 2 |  |
 |
17 | 87 |
| 3 | 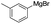 |
 |
15 | 93 |
| 4 |  |
 |
15 | 96 |
| 5 |  |
 |
15 | 87 |
| 6 |  |
 |
17 | 86 |
| 7 |  |
 |
15 | 98 |
| 8 |  |
 |
24 | 61c |
| 9 |  |
 |
15 | 86 |
| 10 |  |
 |
15 | 85 |
| 11 |  |
 |
15 | 88 |
| 12 |  |
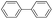 |
15 | 92 |
| 13 |  |
 |
16 | 86 |
| 14 |  |
 |
15 | 89 |
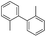
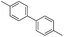
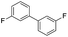
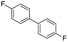
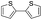
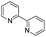
To prove the generality of Ni(OAc)2 as a catalyst in the homo-coupling of Grignard reagents, various substrates were investigated. Optimized reaction conditions17 were next applied to prepare a variety of homo-coupled products. For the aryl Grignard reagents having electron-poor or -rich groups on benzene ring (Table 4, entries 2–7), the coupling reactions provided high yields (85–97%). The steric hindrance also played an important role (Table 4, entries 4 vs. 2 and 7 vs. 6). The longer reaction time was required for the reaction with benzylmagnesium bromide, giving relatively low yields (58%) (Table 4, entry 8). Heteroarylmagnesium bromide with Ni(OAc)2 as a catalyst provided somewhat lower yields than that with Pd(OAc)2 (Table 4, entries 9–11). Similar yields were obtained with arylmagnesium chloride (Table 4, entries 12–14).
| Entry | Grignard reagent | Product | t (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: Grignard reagent (0.3 mmol), Ni(OAc)2 (10 mol%), Ag2O (0.3 mmol, 1.0 equiv.), CH2Cl2 (1.5 mL), temperature (25 °C).b Isolated yield.c 13% yield was obtained in the absence of Ni(OAc)2. | ||||
| 1 |  |
 |
18 | 95c |
| 2 | 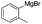 |
 |
20 | 90 |
| 3 |  |
 |
18 | 93 |
| 4 |  |
 |
18 | 97 |
| 5 |  |
 |
17 | 90 |
| 6 |  |
 |
20 | 85 |
| 7 |  |
 |
18 | 92 |
| 8 |  |
 |
24 | 58 |
| 9 |  |
 |
18 | 82 |
| 10 |  |
 |
18 | 77 |
| 11 |  |
 |
18 | 81 |
| 12 |  |
 |
18 | 83 |
| 13 |  |
 |
18 | 86 |
| 14 |  |
 |
18 | 90 |
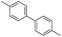
To demonstrate the efficiency and scope of the method with CuI as a catalyst,18 we applied the catalytic system to a variety of Grignard reagents (Table 5). Various substrates including arylmagnesium bromide possessing methyl, methoxy and fluro groups, heteroarylmagnesium bromide, and arylmagnesium chloride were smoothly converted to the desired products in good to excellent yields (Table 5, entries 1–7 and 9–14). Treatment of benzylmagnesium bromide also provided 57% yield at 120 °C (Table 5, entry 8).
| Entry | Grignard reagent | Product | t (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: Grignard reagent (0.3 mmol), CuI (15 mol%), toluene (2.0 mL), temperature (100 °C), air.b Isolated yield.c 120 °C was used. | ||||
| 1 |  |
 |
15 | 95 |
| 2 |  |
 |
18 | 95 |
| 3 |  |
 |
15 | 95 |
| 4 | 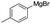 |
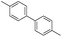 |
15 | 96 |
| 5 |  |
 |
15 | 95 |
| 6 |  |
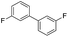 |
18 | 86 |
| 7 |  |
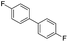 |
18 | 98 |
| 8 |  |
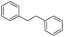 |
24 | 57c |
| 9 |  |
 |
15 | 88 |
| 10 | 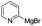 |
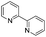 |
15 | 82 |
| 11 |  |
 |
15 | 87 |
| 12 |  |
 |
15 | 89 |
| 13 |  |
 |
16 | 79 |
| 14 |  |
 |
16 | 84 |
Nano Fe3O4 was also found to be a good catalyst.19 Various Grignard reagents turned out to be suitable substrates and worked well (Table 6, entries 1–5 and 9–14) even though the materials bearing electron-withdrawing groups (Table 6, entries 6 and 7). Benzylmagnesium bromide could afford the corresponding product in 50% yield (Table 6, entry 8).
| Entry | Grignard reagent | Product | t (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: Grignard reagent (0.3 mmol), nano-sized Fe3O4 (10 mol%), AgNO3 (0.36 mmol, 1.2 equiv.), toluene (2.0 mL), temperature (100 °C).b Isolated yield.c 9% yield was obtained in the absence of Nano-Fe3O4.d 120 °C was used. | ||||
| 1 | 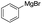 |
 |
20 | 93c |
| 2 |  |
 |
24 | 87 |
| 3 |  |
 |
20 | 89 |
| 4 |  |
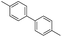 |
20 | 94 |
| 5 |  |
 |
20 | 90 |
| 6 |  |
 |
18 | 76 |
| 7 |  |
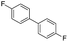 |
18 | 83 |
| 8 |  |
 |
24 | 50d |
| 9 |  |
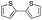 |
18 | 80 |
| 10 | 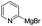 |
 |
20 | 77 |
| 11 |  |
 |
20 | 83 |
| 12 |  |
 |
20 | 86 |
| 13 |  |
 |
20 | 82 |
| 14 |  |
 |
20 | 87 |
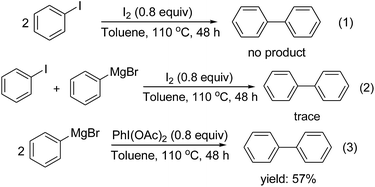
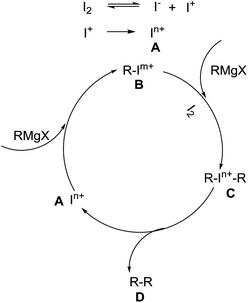
To clarify the possible reaction mechanism I2-promoted, the homocoupling of iodobenzene with the I2/toluene system, cross coupling of iodobenzene with phenyl magnesium bromide in the presence of I2, and phenyl magnesium bromide with PhI(OAc)2/toluene system were carried out (Scheme 1). The experimental results showed that the reaction process may not involve the generation of an iodobenzene (eqn (1) and (2)). The fact that biphenyl was observed in 57% yield when PhI(OAc)2 was used instead of I2 indicated that hypervalent iodine may play an important role in this process, which suggested that iodine serves not only as the promoter but also as the oxidant (eqn (3)). On the basis of the observations and reported literatures,20 a possible mechanism is postulated as follows (Scheme 2): partial iodine may be first transformed into some hypervalent iodine A in the reaction system. Then a low-valent iodine species B is formed through reduction of A using the Grignard reagent as a strong reducing agent. Subsequently, the species B reacts with the Grignard reagent in the presence of I2 as an oxidant to generate intermediate C, which gives the homo-coupling product D by reductive elimination and releases A.
In conclusion, we have described the homo-coupling reactions of various Grignard reagents in the presence of Pd(OAc)2, Ni(OAC)2, CuI, nano-Fe3O4, and I2, respectively. The five synthetic methods worked very well and were applicable to the homo-coupling of various aryl and heteroaryl Grignard reagents. Similar good yields were obtained regarding nano-Fe3O4, and I2. Pd-, Ni-, and Cu-based catalyst systems provided higher yields than them. Notably, the first two systems are more green.
Notes and references
- (a) G. Bringmann, A. J. P. Mortimer, P. A. Keller, M. J. Gresser, J. Garner and M. Breuning, Angew. Chem., Int. Ed., 2005, 44, 5384 CrossRef CAS PubMed; (b) M. G. Organ, M. Abdel-Hadi, S. Avola, N. Hadei, J. Nasielski, C. J. O'Brien and C. Valente, Chem.–Eur. J., 2006, 13, 150 CrossRef PubMed; (c) G. Bringmann, T. Gulder, T. A. M. Gulder and M. Breuning, Chem. Rev., 2011, 111, 563 CrossRef CAS PubMed; (d) M. C. Kozlowski, B. J. Morgan and E. C. Linton, Chem. Soc. Rev., 2009, 38, 3193 RSC; (e) J. Buter, D. Heijnen, C. Vila, V. Hornillos, E. Otten, M. Giannerini, A. J. Minnaard and B. L. Feringa, Angew. Chem., Int. Ed., 2016, 55, 3620 CrossRef CAS PubMed.
- (a) M. S. Kharasch and E. K. Fields, J. Am. Chem. Soc., 1941, 63, 2316 CrossRef CAS; (b) H. Gilman and M. Lichtenwalter, J. Am. Chem. Soc., 1939, 61, 957 CrossRef CAS.
- (a) A. McKillop, L. F. Elsom and E. C. Taylor, J. Am. Chem. Soc., 1968, 90, 2423 CrossRef CAS; (b) A. Mckillop, L. F. Elsom and E. C. Taylor, Tetrahedron, 1970, 26, 4041 CrossRef CAS.
- A. Inoue, K. Kitagawa, H. Shinokubo and K. Oshima, Tetrahedron, 2000, 56, 9601 CrossRef CAS.
- (a) T. Ishikawa, A. Ogawa and T. Hirao, Organometallics, 1998, 17, 5713 CrossRef CAS; (b) C. C. Vernon, J. Am. Chem. Soc., 1931, 53, 3831 CrossRef CAS.
- (a) J. Krizewsky and E. E. Turner, J. Chem. Soc., Trans., 1919, 115, 559 RSC; (b) J. I. Lee, J. Korean Chem. Soc., 2005, 49, 117 CrossRef CAS; (c) D. S. Surry, X. Su, D. J. Fox, V. Franckevicius, S. J. F. Macdonald and D. R. Spring, Angew. Chem., Int. Ed., 2005, 44, 1870 CrossRef CAS PubMed.
- (a) For CrCl3: G. M. Bennett and E. E. Turner, J. Chem. Soc., Trans., 1914, 105, 1057 RSC; (b) For AgBr: J. H. Gardner and P. Borgstrom, J. Am. Chem. Soc., 1929, 51, 3375 CrossRef CAS; (c) For some other metals see: ref. 2b.
- For reactions on transition-metal-free oxidative homocoupling using organic oxidants as mediators. See: (a) M. S. Maji and A. Studer, Synthesis, 2009, 2467 CAS; (b) M. S. Maji, T. Pfeifer and A. Studer, Chem.–Eur. J., 2010, 16, 5872 CrossRef CAS PubMed; (c) T. Nishiyama, T. Seshita, H. Shodai, K. Aoki, H. Kameyama and K. Komura, Chem. Lett., 1996, 25, 549 CrossRef; (d) J.-W. Cheng and F.-T. Luo, Tetrahedron Lett., 1988, 29, 1293 CrossRef CAS; (e) A. Krasovskiy, A. Tishkov, V. Amo, H. Mayr and P. Knochel, Angew. Chem., Int. Ed., 2006, 45, 5010 CrossRef CAS PubMed; (f) T. Amaya, R. Suzuki and T. Hirao, Chem.–Eur. J., 2014, 20, 653 CrossRef CAS PubMed; (g) M. S. Maji, T. Pfeifer and A. Studer, Angew. Chem., Int. Ed., 2008, 47, 9547 CrossRef CAS PubMed.
- (a) T. Hatakeyama and M. Nakamura, J. Am. Chem. Soc., 2007, 129, 9844 CrossRef CAS PubMed; (b) A. K. Steib, O. M. Kuzmina, S. Fernandez, D. Flubacher and P. Knochel, J. Am. Chem. Soc., 2013, 135, 15346 CrossRef CAS PubMed; (c) J. Terao and N. Kambe, Acc. Chem. Res., 2008, 41, 1545 CrossRef CAS PubMed; (d) G. Cahiez, O. Gager and F. Lecomte, Org. Lett., 2008, 10, 5255 CrossRef CAS PubMed; (e) M. Nakamura, K. Matsuo, S. Ito and E. Nakamura, J. Am. Chem. Soc., 2004, 126, 3686 CrossRef CAS PubMed; (f) J. Terao, H. Watanabe, A. Ikumi, H. Kuniyasu and N. Kambe, J. Am. Chem. Soc., 2002, 124, 4222 CrossRef CAS PubMed; (g) T. Hatakeyama, S. Hashimoto, K. Ishizuka and M. Nakamura, J. Am. Chem. Soc., 2009, 131, 11949 CrossRef CAS PubMed; (h) L. Ackermann, R. Born, J. H. Spatz and D. Meyer, Angew. Chem., Int. Ed., 2005, 44, 7216 CrossRef CAS PubMed.
- (a) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS PubMed; (b) Z. Zhou and W. Xue, J. Organomet. Chem., 2009, 694, 599 CrossRef CAS; (c) G. Cahiez, A. Moyeux, J. Buendia and C. Duplais, J. Am. Chem. Soc., 2007, 129, 13788 CrossRef CAS PubMed.
- (a) T. Nagano and T. Hayashi, Org. Lett., 2005, 7, 491 CrossRef CAS PubMed; (b) G. Cahiez, C. Chaboche, F. Mahuteau-Betzer and M. Ahr, Org. Lett., 2005, 7, 1943 CrossRef CAS PubMed; (c) W. Liu and A. Lei, Tetrahedron Lett., 2008, 49, 610 CrossRef CAS; (d) Y. Moglie, E. Mascaró, F. Nador, C. Vitale and G. Radivoy, Synth. Commun., 2008, 38, 3861 CrossRef CAS; (e) J. Wu, W. Dai, J. H. Farnaby, N. Hazari, J. J. L. Roy, V. Mereacre, M. Murugesu, A. K. Powellc and M. K. Takasea, Dalton Trans., 2013, 42, 7404 RSC; (f) G. Kiefer, L. Jeanbourquin and K. Severin, Angew. Chem., Int. Ed., 2013, 52, 6302 CrossRef CAS PubMed; (g) See ref. 10c.
- (a) See ref. 11f; (b) Z. Mo, Y. Li, H. K. Lee and L. Deng, Organometallics, 2011, 30, 4687 CrossRef CAS; (c) G. Cahiez and A. Moyeux, Chem. Rev., 2010, 110, 1435 CrossRef CAS PubMed.
- P. I. Aparna and B. R. Bhat, J. Mol. Catal. A: Chem., 2012, 358, 73 CrossRef CAS.
- (a) See ref. 11f; (b) Y. Zhu, T. Xiong, W. Han and Y. Shi, Org. Lett., 2014, 16, 6144 CrossRef CAS PubMed.
- Extensive screening showed that the optimized reaction conditions were 0.3 mmol Grignard reagent, 10 mol% Pd(OAc)2 and 1.0 equiv. of LiClO4 in toluene under N2 atmosphere at 100 °C. (See Table 1 in ESI†).
- LiClO4 served as an oxidant.
- The optimal reaction conditions screened were 0.3 mmol Grignard reagent, 10 mol% Ni(OAc)2 and 1.0 equiv. of Ag2O which was used as an oxidant in 1.5 mL CH2Cl2 under N2 atmosphere at 25 °C. (See Table 2 in ESI†).
- The optimized reaction conditions were 0.3 mmol Grignard reagent and 15 mol% CuI in 2.0 mL toluene under air atmosphere at 100 °C. (See Table 3 in ESI†).
- The optimal reaction conditions were 0.3 mmol Grignard reagent, 10 mol% nano-Fe3O4 and 1.2 equiv. of AgNO3 utilized as an oxidant in 2.0 mL toluene under N2 atmosphere at 100 °C. (See Table 4 in ESI†).
- J. Mao, Q. Hua, G. Xie, Z. Yao and D. Shi, Eur. J. Org. Chem., 2009, 2262 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17859f |
| This journal is © The Royal Society of Chemistry 2016 |