A facile one-pot multi-component synthesis of novel adamantine substituted imidazo[1,2-a]pyridine derivatives: identification and structure–activity relationship study of their anti-HIV-1 activity
Tazeem†
ab,
Xin Han†ab,
Qingjun Zhouc,
Jingchen Weid,
Po Tiena,
Guichun Yangc,
Shuwen Wu*a and
Chune Dong*b
aState Key Laboratory of Virology, College of Life Sciences, Wuhan University, Wuhan 430072, China. E-mail: shuwenwu@hotmail.com
bHubei Province Engineering and Technology Research Center for Fluorinated Pharmaceuticals, Wuhan University School of Pharmaceutical Sciences, Wuhan 430071, China. E-mail: cdong@whu.edu.cn
cHubei Collaborative Innovation Center for Advanced Organochemical Materials, Ministry of Education Key Laboratory for the Synthesis and Application of Organic Functional Molecules, Hubei University, Wuhan 430062, China
dDepartment of Pharmacology, Guilin Medical University, Guilin, Guangxi 541004, China
First published on 29th September 2016
Abstract
In this study, a series of adamantine substituted imidazo[1,2-a]pyridine derivatives were designed and synthesized through a one-pot multi-component Groebke–Blackburn–Bienaymé reaction. Their anti-HIV activities were evaluated using an HIV-1IIIB/TZM-bl indicator cell culture system, in which compounds 19d, 19e and 19m were found to be the most potent inhibitors with EC50 values of 0.048, 0.061 and 0.077 μM, respectively. Furthermore, the modeling results provided valuable insight into how compound 19d gave good efficacy against HIV-1 cells.
Before the discovery of azidothymidine (AZT) in 1987, acquired immunodeficiency syndrome (AIDS) was considered to be an incurable and potentially fatal disease. AZT, known as a nucleoside reverse transcriptase inhibitor (NRTI), was the first breakthrough in AIDS therapy.1 At that time, it opened the doors to new hope for the treatment of HIV-1 infection and converted an incurable disease to a curable one. Since then, several types of anti-HIV-1 drugs have been developed. For example, saquinavir 1 and nevirapine (NVP) 2 were used as a protease inhibitor and non-nucleoside reverse transcriptase inhibitor (NNRTI), respectively.2 This symbolized significant progress in HIV-1 treatment. Thereafter, delavirdine (DLV) 3, efavirenz (EFV) 4, etravirine 5 and rilpivirine 6, as the new generation of NNRTIs, have already been approved for use in HIV-1 treatment (Scheme 1).3–5
Nowadays, HIV-1 infection can be controlled by the use of multiple antiretroviral drugs. There are several classes of antiretroviral drugs available for the treatment of HIV-1 infection. Among them, highly active antiretroviral therapy (HAART) is one of the best treatments targeting the virus at different stages of its life-cycle.6 However, long-term use of HAART by patients triggers the emergence of drug-resistant and drug-related adverse effects.7,8 Thus, the identification of novel anti-HIV-1 agents with antiviral activity against drug-resistant strains of HIV-1 is critically urgent.
As mentioned above, NNRTIs play an important role in the HARRT. To overcome the problem of drug resistance, several NNRTIs have been recently developed such as rilpivirine,9 fosdevirine (GSK2248761) 7,10,11 lersivirine (UK-453061) 8,12 RDEA806 913 and are currently in clinical trial (Scheme 2). In 2010, pyridine based NNRTIs was reported having strong inhibitory effect on polymerase activity.14
Recently, Gomha and co-workers reported pyrazolo[4,3-d]isoxazole derivatives were screened for their antiviral activities against two viral strains of HIV-1 (RF and IIIB) with EC50 value up to 0.25 nM.15 In 2012, Wang and co-workers developed 6-aryluracils which exhibited highly potent anti-HIV-1 activity against both on HIV-1SF33 and HIV-1A17 replication in MT4 cell.16 In 2015, Frey and co-workers developed analogues of the catechol diether series having picomolar activity against HIV strains with wild-type RT Y181C and K103N/Y181C variants.17
N-Fused heterocycles and adamantane are components of the important classes of molecules that were found in a variety of natural products and biologically active compounds. In particular, imidazo[1,2-a]pyridines derivatives, which can be efficiently synthesized through one-pot procedure from 2-aminopyridines and aldehydes,18–25 have drawn much attention due to their significant pharmaceutical properties such as anticancer,26–29 antivirals,30–33 antimicrobials,34–37 anti-Parkinson,38 antimutagenics,39 antihypoxia,40 anticonvulsants,41 antisecretory,42,43 and as antiinflamatories etc.44 In 2011, Dahan-Farkas and co-workers developed a range of 6-substituted imidazo[1,2-a]pyridines as anti-cancer agents against the colon cancer cell lines HT-29 and Caco-2 with moderate IC50 values, e.g. 10 in Scheme 3.45 At the same year, Bode and co-workers reported a library of compounds with imidazo[1,2-a]pyridines skeleton such as 11 which was prepared using the Groebke reaction with moderate anti-HIV-1 activity of 14.47 μM (Scheme 3).46
On the other hand, there are number of adamantly bearing molecules reported in the literature which have long been known for their anti-HIV-1 activity.47 Kolocouris reported the anti-HIV-1 activity of compound 12 in cell culture.48,49 Other adamantine derivatives, such as 2-(1-adamantyl) piperidines 13 and 14, were developed by Stamatiou and co-workers exhibited modest anti-HIV activities (Scheme 4).50,51 Burstein and co-workers evaluated newly developed antiviral compounds consisting of an adamantine moiety and a water-soluble polyanionic matrix which could inhibit HIV-1 infection in lymphoblastoid cells, HeLa CD4 + β-galactosidase (MAGI) cells with IC50 value up to 93 μM.52 Balzarini group reported that the thiazolidin-4-one derivative 15 bearing a adamantyl substituent exhibited modest anti-HIV-1 activity (EC50 = 0.67 μM).53
During the past few years,54–59 we have focused on the development of novel non-nucleoside reverse transcriptase inhibitors, a large amount of small molecule NNRTIs, such as indole-based α-amino acids,60 indole-based trifluoropropanoates61 and halolactones61 were developed. In order to continue pursuing in this field, we envisioned that incorporation of imidazo[1,2-a]pyridines62 and adamantine63,64 moieties in one molecule could enhance their anti-HIV activity. Herein, the imidazo[1,2-a]pyridines derivatives bearing adamantane tail were designed and synthesized, and their anti-HIV activities were evaluated using a HIV-1IIIB/TZM-bl indicator cell culture system.
Results and discussion
Chemistry
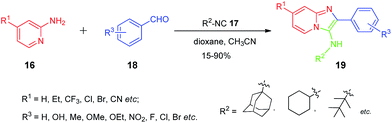
The new series of imidazo[1,2-α]pyridine derivatives 19 and 21 were designed as shown below in order to find-out the effect of different substituents on the anti-HIV-1 activity. The compounds imidazo[1,2-a]pyridines 19 were prepared according to previously reported method65 and the procedure was shown in Scheme 5. The 2-aminopyridine, isonitriles and aldehydes were reacted in one-pot to form the desired products imidazo[1,2-a]pyridines derivatives in good yields.Structure–activity relationship analysis
The compounds were assayed for their anti-HIV-1 activity by using a HIV-1IIIB/TZM-bl indicator cell culture system. All of the newly synthesized compounds were tested, and the results are summarized in Tables 1 and 2. Most of the compounds showed moderate to good inhibitory activities against HIV-1IIIB in a HIV-1IIIB/TZM-bl indicator cell culture system. Out of all these compounds 19d, 19e and 19m were found to be the most potent inhibitors with EC50 values of 0.048, 0.061 and 0.077 μM, respectively. It is worth outlining that the substituents R1, R2 and R3 have significant effect on the anti-HIV-1 activity. Compound 19g exhibited better anti-HIV-1 activity than 19b and 19c, in which the methoxyl group substituted on the phenyl ring, giving a moderate EC50 of 3.52 μM in TZM-bl cells. However, when the substitution occurred at para position of the phenyl ring with halogen atoms, the potency increased accordingly. For example, compound 19t gave good EC50 value 0.30 μM. It was also observed that the hydroxyl group at para position of phenyl ring had better influence on anti-HIV activity than meta position in the cases of 19f and 19y with EC50 0.163 and 0.447 μM, respectively. Further study revealed that with the insertion of ethoxyl and nitro groups at ortho position of phenyl ring induced more inhibition than their analogues (19d and 19e) with EC50 values 0.048 and 0.061 μM. On the other hand, chloro group substituted at meta position (19x) had better effect on the anti-HIV activity as compared to the other groups such as hydroxyl, fluoro and bromo (19h, 19g and 19v). It was also observed that the insertion of electron withdrawing such as chloro, bromo, nitrile and trifluoromethyl groups at R1 diminished the activity of compounds as compared with the parent analogues. It was found that electron withdrawing group diminished the activity of compounds 19j–19s as compared to the corresponding parent analogues 19d and 19e. A dramatic decrease was observed in the activity from 0.048 μM to 3.705 μM in the case of 19r having trifluoromethyl group at R1 position. When the bromo (19l) and nitrile (19n) groups were substituted at the R1 position, the EC50 value shifted to 1.382 and 1.633 μM, respectively; whereas the chloro and ethyl substituted derivatives showed moderate activity. Furthermore, when the cytotoxicity (CC50 values) was evaluated, most of the compounds showed moderate cytotoxicity, especially, compounds 19d and 19e showed SI (selectivity index) values of 426.6 and 661.3, respectively.| Entry | Compd | R1 | R2 | R3 | EC50b (μM) | CC50c (μM) | SId |
|---|---|---|---|---|---|---|---|
| a All data are mean values ± standard deviation for at least three independent experiments.b EC50: effective concentration (μM) for 50% inhibition of HIV-1 (HIV-1IIIB strain) as evaluated with the luciferase activity in TZM-bl cells.c CC50: cytotoxic concentration (μM) to induce 50% death of noninfected cells, as evaluated with the MTT method in TZM-bl cells.d SI: selectivity index calculated as CC50/EC50 ratio.e ND: not determined.f Not calculated because the EC50 was too high. | |||||||
| 1 | 19a | H |  |
4-OCH3 | 32.450 ± 3.530 | 54.35 ± 15.26 | 1.7 |
| 2 | 19b | H | 3,4-(OCH3)2 | 5.990 ± 1.670 | 29.73 ± 8.92 | 5.0 | |
| 3 | 19c | H | H | 3.520 ± 1.580 | 94.62 ± 21.25 | 26.9 | |
| 4 | 19d | H | 2-NO2-4-OH | 0.048 ± 0.017 | 20.52 ± 7.17 | 426.6 | |
| 5 | 19e | H | 2-OEt-4-OH | 0.061 ± 0.009 | 40.14 ± 12.88 | 661.3 | |
| 6 | 19f | H | 4-OH | 0.163 ± 0.063 | 22.25 ± 10.01 | 136.5 | |
| 7 | 19g | H | 3-F-4-OH | 0.130 ± 0.045 | 22.28 ± 10.87 | 171.4 | |
| 8 | 19h | H | 3-OH-4-OH | 1.34 ± 0.57 | 76.26 ± 20.21 | 56.9 | |
| 9 | 19i | H | 3,4-OC2H2O– | 0.316 ± 0.110 | 10.22 ± 4.73 | 32.3 | |
| 10 | 19j | Cl | 2-OEt-4-OH | 0.303 ± 0.082 | 10.52 ± 5.26 | 34.7 | |
| 11 | 19k | Cl | 4-OH | 0.271 ± 0.098 | 22.13 ± 8.39 | 81.7 | |
| 12 | 19l | Br | 2-OEt-4-OH | 1.382 ± 0.500 | 34.85 ± 11.82 | 25.2 | |
| 13 | 19m | CF3 | 2-OEt-4-OH | 0.077 ± 0.029 | 117.83 ± 7.64 | 252.5 | |
| 14 | 19n | CN | 2-OEt-4-OH | 1.633 ± 0.590 | 20.32 ± 6.77 | 12.4 | |
| 15 | 19o | Br | 4-OH | 0.289 ± 0.132 | 10.27 ± 5.25 | 35.5 | |
| 16 | 19p | Et | 2-NO2-4-OH | 0.321 ± 0.154 | 15.74 ± 7.17 | 49.0 | |
| 17 | 19q | Br | 2-NO2-4-OH | 0.331 ± 0.095 | 8.69 ± 3.31 | 26.3 | |
| 18 | 19r | CF3 | 2-NO2-4-OH | 3.705 ± 1.050 | 15.25 ± 6.77 | 14.5 | |
| 19 | 19s | CN | 2-NO2-4-OH | 0.253 ± 0.098 | 20.04 ± 6.06 | 79.2 | |
| 20 | 19t | H | 4-Br | 0.300 ± 0.113 | 21.80 ± 7.34 | 72.7 | |
| 21 | 19u | H | 3-OEt-4-OH | 1.330 ± 0.066 | 13.89 ± 6.69 | 210.3 | |
| 22 | 19v | H | 3-Br-4-OH | 0.303 ± 0.150 | 36.52 ± 11.87 | 120.5 | |
| 23 | 19w | H | 2-F-4-OH | 1.330 ± 0.627 | 24.40 ± 9.81 | 18.4 | |
| 24 | 19x | H | 3-Cl-4-OH | 0.077 ± 0.048 | 17.30 ± 6.10 | 225.0 | |
| 25 | 19y | H | 3-Me-4-OH | 0.447 ± 0.195 | 25.73 ± 10.18 | 57.6 | |
| 26 | 19z | H |  |
2-NO2-4-OH | >100 | NDe | —f |
| 27 | 19z1 | H |  |
2-NO2-4-OH | >100 | ND | — |
| 28 | 2 |  |
0.042 ± 0.014 | 144.3 ± 42.6 | 3435.72 | ||
| Entry | Compd | R4 | R5 | EC50b (μM) | CC50c (μM) | SId |
|---|---|---|---|---|---|---|
| a All data are mean values ± standard deviation for at least three independent experiments.b EC50: effective concentration (μM) for 50% inhibition of HIV-1 (HIV-1IIIB strain) as evaluated with the luciferase activity in TZM-bl cells.c CC50: cytotoxic concentration (μM) to induce 50% death of noninfected cells, as evaluated with the MTT method in TZM-bl cells.d SI: selectivity index calculated as CC50/EC50 ratio. | ||||||
| 1 | 21a | H | 5-F | 12.125 ± 3.300 | 141.74 ± 23.25 | 11.7 |
| 2 | 21b | H | 5-OCH3 | 4.410 ± 1.970 | 155.33 ± 30.54 | 35.2 |
| 3 | 21c | H | H | 1.590 ± 0.729 | 62.74 ± 18.52 | 39.5 |
| 4 | 21d | H | 5-Me | 0.384 ± 0.058 | 21.18 ± 9.33 | 55.2 |
| 5 | 21e | H | 7-Me | 0.452 ± 0.179 | 15.63 ± 8.57 | 34.6 |
| 6 | 21f | H | 5-CN | 0.353 ± 0.137 | 10.30 ± 4.17 | 29.2 |
| 7 | 21g | 1-Me | H | 0.268 ± 0.068 | 17.15 ± 9.33 | 64.0 |
| 8 | 21h | H | 5-Cl | 0.688 ± 0.213 | 7.69 ± 3.84 | 11.3 |
| 9 | 21i | H | 6-Me | 0.601 ± 0.166 | 5.80 ± 2.01 | 9.7 |
| 10 | 21j |  |
0.534 ± 0.164 | 16.73 ± 7.32 | 31.3 | |
| 11 | 2 |  |
0.042 ± 0.014 | 144.3 ± 42.6 | 3435.72 | |
The structural–activity relationship analysis of alternate substituents present on the phenyl ring and imidazo[1,2-a]pyridine bearing adamantine is presented in Fig. 1. The results demonstrate that substituted phenyl ring at the 4-position has better HIV-1 inhibitory activity, for example compound 19t with Br at the 4-position of the phenyl ring gives lower EC50 value than compound 19c. Furthermore, the OH group which can form a hydrogen bonding interaction with residue Glu 238 is the best substituent for the anti-HIV-1 activity, such as compound 19f, this can be explained by the molecular modeling studies in Fig. 2. For other substituents at the 2- or 3-position of the phenyl ring, we found that compounds 19d and 19e with 2-NO2-4-OH and 2-OEt-4-OH substituents at the phenyl ring respectively, gave the best anti-HIV-1 activities as compared with the reference NVP 2, in which nitro group formed two hydrogen bondings with residue Lys 101. Decreased HIV-1 inhibitory activity occurred in the presence of 3-OEt-4-OH at the phenyl ring, while 3-Cl-4-OH substituted phenyl ring gave decreased but considerable activity as compared with compounds 19d and 19e. Finally, we researched on the groups at the imidazo[1,2-a]pyridine ring (19j–s) and the results showed that substituted imidazo[1,2-a]pyridine ring generally gave lower anti-HIV-1 activities, except compound 19m showed comparable activity to those of the best compounds 19d and 19e of the series. It should also be noted that the compounds 19z and 19z1 bearing the same imidazo[1,2-a]pyridine structure, but lacking the adamantyl moiety, was devoid of antiviral activity (entries 26–27).
 | ||
| Fig. 1 Graphical representation of the effect of substituents present on the imidazo[1,2-a]pyridine and phenyl rings on the anti-HIV-1 activity. | ||
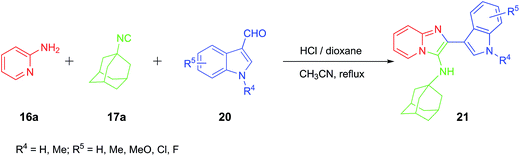
Meanwhile, indole is also one of the most used bioactive components for drug discovery, including anti-HIV-1 agent.65 Therefore, indole based imidazo[1,2-a]pyridine (21) derivatives were also synthesized and their anti-HIV activities were evaluated, the results were summarized in Table 2. Surprisingly, when the phenyl ring was replaced with the indole ring, decreased potency was usually observed and the substitution plays a major role on the anti-HIV-1 activity. Compounds 21g, in which the indole ring substituted by methyl group at N-1 position, showed the most potent activity (entry 7).
Among them, 21f showed moderate activity with IC50 value up to 0.353 μM, in contrast, 21a, 21b and 21h, having fluoro, methoxyl, and chloro group at the same position gave lower activity.
Moreover, in order to ascertain whether the compounds were also inhibitory in primary T-lymphocyte cells using virus strains that belong to different clades of HIV-1,66 compounds 19d and 19e were included in this study (Table 3). These compounds showed good inhibitory activity against virus members that belonged to clade A, clade B, clade C and group O. The clade viruses (92UG029 and BCF02) were X-tropic, while the clade viruses (92US657 and 93IN101) were R-tropic. These data indicated that this class of compounds possess a broad spectrum of anti-HIV-1 activity.
| EC50a (μM) | |||||
|---|---|---|---|---|---|
| Entry | Compd | Clade A (92UG029) | Clade B (92US657) | Clade C (93IN101) | Group O (BCF02) |
| a EC50: 50% effective concentration or compound concentration required to inhibit HIV-1 p24 production in virus-infected PBMC. Data are the mean of two independent experiments. | |||||
| 1 | 19d | 0.057 | 0.065 | 0.092 | 0.084 |
| 2 | 19e | 0.074 | 0.086 | 0.124 | 0.136 |
Molecular modeling studies
In order to further understanding the structure–binding relationships of imidazo[1,2-a]pyridine derivatives in the non-nucleoside binding site (NNBS) of RT, we took a molecular modeling approach. In the modeling study, we chose the crystal structure of WT RT NNBS with (2-(((2-(3,4-dihydroquinolin-1(2H)-yl)-2-oxoethyl)(methyl)amino)-methyl)quinazolin-4(1H)-one) (PDB: 4KFB)67–69 and compound 19d as the receptor and ligand, respectively. AutoDock Vina70 was chosen to study the binding modes of compound 19d and the result showed excellent binding score (−12.41 kcal mol−1) in the NNBS of RT where three hydrogen bonding interactions were formed between the nitro group and residue Lys 101, OH group and residue Glu 238 which can explain that compounds with NO2 group gave better activities. In the proposed structure of protein-19d complex, adamantine tail was buried deep in the site formed by residues Leu 100-Lys 102-Lys 103-Val 106-Phe 227-Leu 234-Pro 236-Tyr 318. The imidazo[1,2-a]pyridine ring forms several π–π interactions with residues Tyr 181, Tyr 188, and Trp 229 in the hydrophobic pocket, which is mainly formed by the side chains of Tyr181, Tyr 188, Phe 227, Trp 229, and Leu 234.Conclusion
The biological properties of the imidazo[1,2-a]pyridine class of compounds have drawn much attention in recent years, however their effect on HIV-1IIIB/TZM-bl indicator cell culture system has not yet been established. In the present study, a number of imidazo[1,2-a]pyridin-3-amines were synthesised in a one-pot procedure from appropriate aldehydes, isocyanides and 2-aminopyridines using the Groebke reaction and their anti-HIV activities were evaluated. The SAR results indicate that the NO2 and hydroxyl group at ortho and para position of phenyl ring, played important role in anti-HIV-1 activity. Further study on mechanism of action of these compounds is currently under way.Experimental section
Materials and methods
Unless otherwise noted, reagents and materials were obtained from commercial suppliers and were used without further purification. Reactions were monitored by thin layer chromatography (TLC) and column chromatography purification was performed using 230–400 mesh silica gel. NMR spectra were measured on Bruker DRX and DMX spectrometers at 400 MHz for 1H spectra and at 100 MHz for 13C spectra and calibrated from the residual solvent signal. All final products were characterized by 1H NMR, 13C NMR and MS analyses.Live subject statement
All experiments were performed in compliance with the relevant laws and institutional guidelines, and the institutional committee (the study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University. All experiments were performed in accordance with ethical standards laid down in the Declaration of Helsinki). The informed consent was obtained for any experimentation with human subjects.Representative procedure for the synthesis of N-(1-alkyl)-2-substituted aryl-imidazo[1,2-a]substitutedpyridin-3-amine 19
To the solution of substituted 2-aminopyridine (0.25 mmol) in anhydrous acetonitrile, were added aldehyde (0.28 mmol), a catalytic amount of 4 N HCl/dioxane (10 μL) and isonitrile (0.24 mmol). The reaction mixture was refluxed for 2–6 hours and the reaction was monitored by thin layer chromatography (TLC). After the reaction mixture was cooled to room temperature, solvent was removed and the residue was purified through column chromatography.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1298 (C–N), 1193 (C–OCH3). 1H NMR (400 MHz, CDCl3) δ 8.24 (d, J = 6.9 Hz, 1H, Ar-H), 7.92–7.85 (m, 2H, Ar-H), 7.51 (d, J = 9.0 Hz, 1H, Ar-H), 7.09 (ddd, J = 8.9, 6.6, 1.2 Hz, 1H, Ar-H), 7.00–6.93 (m, 2H, Ar-H), 6.73 (td, J = 6.8, 0.9 Hz, 1H, Ar-H), 3.85 (s, 3H, OCH3), 3.02 (s, 1H, NH), 1.93–1.44 (m, 15H, adamantyl-H). 13C NMR (100 MHz, CDCl3) δ 158.05, 141.91, 139.40, 129.30, 127.88, 121.83, 117.02, 113.63, 111.05, 56.24, 55.24, 43.85, 36.16, 29.66. HRMS (ESI) calcd for C24H28N3O [M + H]+ 374.2232, found 374.2206.
N), 1298 (C–N), 1193 (C–OCH3). 1H NMR (400 MHz, CDCl3) δ 8.24 (d, J = 6.9 Hz, 1H, Ar-H), 7.92–7.85 (m, 2H, Ar-H), 7.51 (d, J = 9.0 Hz, 1H, Ar-H), 7.09 (ddd, J = 8.9, 6.6, 1.2 Hz, 1H, Ar-H), 7.00–6.93 (m, 2H, Ar-H), 6.73 (td, J = 6.8, 0.9 Hz, 1H, Ar-H), 3.85 (s, 3H, OCH3), 3.02 (s, 1H, NH), 1.93–1.44 (m, 15H, adamantyl-H). 13C NMR (100 MHz, CDCl3) δ 158.05, 141.91, 139.40, 129.30, 127.88, 121.83, 117.02, 113.63, 111.05, 56.24, 55.24, 43.85, 36.16, 29.66. HRMS (ESI) calcd for C24H28N3O [M + H]+ 374.2232, found 374.2206.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1284 (C–N), 1190 (C–OCH3). 1H NMR (400 MHz, CDCl3) δ 8.56 (d, J = 6.8 Hz, 1H, Ar-H), 7.78 (d, J = 8.9 Hz, 1H, Ar-H), 7.72–7.65 (m, 2H, Ar-H), 7.45–7.36 (m, 1H, Ar-H), 7.06 (td, J = 6.9, 0.9 Hz, 1H, Ar-H), 6.70 (d, J = 8.5 Hz, 1H, Ar-H), 5.25 (s, 1H, NH), 4.10 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 1.90–1.26 (m, 15H, adamantyl-H). 13C NMR (100 MHz, CDCl3) δ 149.51, 148.34, 136.24, 130.20, 123.25, 121.05, 118.71, 115.27, 111.98, 110.84, 110.42, 56.88, 55.65, 43.95, 35.95, 29.56. HRMS (ESI) calcd for C25H30N3O2 [M + H]+ 404.2338, found 404.2311.
N), 1284 (C–N), 1190 (C–OCH3). 1H NMR (400 MHz, CDCl3) δ 8.56 (d, J = 6.8 Hz, 1H, Ar-H), 7.78 (d, J = 8.9 Hz, 1H, Ar-H), 7.72–7.65 (m, 2H, Ar-H), 7.45–7.36 (m, 1H, Ar-H), 7.06 (td, J = 6.9, 0.9 Hz, 1H, Ar-H), 6.70 (d, J = 8.5 Hz, 1H, Ar-H), 5.25 (s, 1H, NH), 4.10 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 1.90–1.26 (m, 15H, adamantyl-H). 13C NMR (100 MHz, CDCl3) δ 149.51, 148.34, 136.24, 130.20, 123.25, 121.05, 118.71, 115.27, 111.98, 110.84, 110.42, 56.88, 55.65, 43.95, 35.95, 29.56. HRMS (ESI) calcd for C25H30N3O2 [M + H]+ 404.2338, found 404.2311.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1217 (C–N), 1H NMR (400 MHz, CDCl3) δ 8.27 (d, J = 6.9 Hz, 1H, Ar-H), 7.98–7.90 (m, 2H, Ar-H), 7.53 (d, J = 9.0 Hz, 1H, Ar-H), 7.43 (t, J = 7.6 Hz, 2H, Ar-H), 7.35–7.24 (m, 1H, Ar-H), 7.12 (ddd, J = 8.9, 6.6, 1.2 Hz, 1H, Ar-H), 6.76 (td, J = 6.8, 1.0 Hz, 1H, Ar-H), 3.10 (s, 1H, NH), 1.92–1.43 (m, 15H, adamantyl-H). 13C NMR (100 MHz, CDCl3) δ 141.90, 139.29, 135.09, 128.15, 127.36, 117.15, 111.32, 56.68, 43.81, 36.14, 29.65. HRMS (ESI) calcd for C23H26N3 [M + H]+ 344.2127, found 344.2102.
N), 1217 (C–N), 1H NMR (400 MHz, CDCl3) δ 8.27 (d, J = 6.9 Hz, 1H, Ar-H), 7.98–7.90 (m, 2H, Ar-H), 7.53 (d, J = 9.0 Hz, 1H, Ar-H), 7.43 (t, J = 7.6 Hz, 2H, Ar-H), 7.35–7.24 (m, 1H, Ar-H), 7.12 (ddd, J = 8.9, 6.6, 1.2 Hz, 1H, Ar-H), 6.76 (td, J = 6.8, 1.0 Hz, 1H, Ar-H), 3.10 (s, 1H, NH), 1.92–1.43 (m, 15H, adamantyl-H). 13C NMR (100 MHz, CDCl3) δ 141.90, 139.29, 135.09, 128.15, 127.36, 117.15, 111.32, 56.68, 43.81, 36.14, 29.65. HRMS (ESI) calcd for C23H26N3 [M + H]+ 344.2127, found 344.2102.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1230 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.73 (s, 1H, Ar-H), 8.41 (d, J = 6.9 Hz, 1H, Ar-H), 7.84 (d, J = 8.9 Hz, 1H, Ar-H), 7.44 (d, J = 9.0 Hz, 1H, Ar-H), 7.32–7.05 (m, 2H, Ar-H), 6.97–6.74 (m, 2H, Ar-H), 4.39 (s, 1H, NH), 1.84–1.36 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 160.67, 141.41, 140.97, 132.60, 124.13, 123.69, 118.25, 114.36, 111.24, 56.21, 40.06, 35.74, 28.95. HRMS (ESI) calcd for C23H25N4O3 [M + H]+ 405.1927, found 405.1900.
N), 1230 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.73 (s, 1H, Ar-H), 8.41 (d, J = 6.9 Hz, 1H, Ar-H), 7.84 (d, J = 8.9 Hz, 1H, Ar-H), 7.44 (d, J = 9.0 Hz, 1H, Ar-H), 7.32–7.05 (m, 2H, Ar-H), 6.97–6.74 (m, 2H, Ar-H), 4.39 (s, 1H, NH), 1.84–1.36 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 160.67, 141.41, 140.97, 132.60, 124.13, 123.69, 118.25, 114.36, 111.24, 56.21, 40.06, 35.74, 28.95. HRMS (ESI) calcd for C23H25N4O3 [M + H]+ 405.1927, found 405.1900.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1247 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 9.16 (s, 1H, –OH), 8.49 (d, J = 6.9 Hz, 1H, Ar-H), 7.78 (d, J = 1.8 Hz, 1H, Ar-H), 7.62 (dd, J = 8.3, 1.9 Hz, 1H, Ar-H), 7.51 (d, J = 8.9 Hz, 1H, Ar-H), 7.34–7.23 (m, 1H, Ar-H), 6.96 (t, J = 6.5 Hz, 1H, Ar-H), 6.85 (d, J = 8.21 Hz, 1H, Ar-H), 4.71 (s, 1H, NH), 4.11 (q, J = 7.0 Hz, 2H, –CH2–), 1.88–1.36 (m, 18H, adamantyl-H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 146.51, 146.15, 139.79, 125.46, 124.38, 121.96, 120.78, 115.24, 113.14, 111.92, 66.83, 55.80, 43.20, 35.78, 29.02, 14.87. HRMS (ESI) calcd for C25H30N3O2 [M + H]+ 404.2338, found 404.2309.
N), 1247 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 9.16 (s, 1H, –OH), 8.49 (d, J = 6.9 Hz, 1H, Ar-H), 7.78 (d, J = 1.8 Hz, 1H, Ar-H), 7.62 (dd, J = 8.3, 1.9 Hz, 1H, Ar-H), 7.51 (d, J = 8.9 Hz, 1H, Ar-H), 7.34–7.23 (m, 1H, Ar-H), 6.96 (t, J = 6.5 Hz, 1H, Ar-H), 6.85 (d, J = 8.21 Hz, 1H, Ar-H), 4.71 (s, 1H, NH), 4.11 (q, J = 7.0 Hz, 2H, –CH2–), 1.88–1.36 (m, 18H, adamantyl-H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 146.51, 146.15, 139.79, 125.46, 124.38, 121.96, 120.78, 115.24, 113.14, 111.92, 66.83, 55.80, 43.20, 35.78, 29.02, 14.87. HRMS (ESI) calcd for C25H30N3O2 [M + H]+ 404.2338, found 404.2309.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1282 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 9.83 (s, 1H, –OH), 8.62 (d, J = 6.7 Hz, 1H, Ar-H), 7.99 (d, J = 8.5 Hz, 2H, Ar-H), 7.62 (d, J = 8.7 Hz, 1H, Ar-H), 7.48 (t, J = 7.7 Hz, 1H, Ar-H), 7.13 (t, J = 6.7 Hz, 1H, Ar-H), 6.87 (d, J = 8.5 Hz, 2H, Ar-H), 4.83 (s, 1H, NH), 1.88–1.41 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 157.37, 139.10, 129.22, 124.74, 122.99, 122.15, 115.05, 114.34, 112.87, 55.89, 40.05, 35.72, 29.00. HRMS (ESI) calcd for C23H26N3O [M + H]+ 360.2076, found 360.2048.
N), 1282 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 9.83 (s, 1H, –OH), 8.62 (d, J = 6.7 Hz, 1H, Ar-H), 7.99 (d, J = 8.5 Hz, 2H, Ar-H), 7.62 (d, J = 8.7 Hz, 1H, Ar-H), 7.48 (t, J = 7.7 Hz, 1H, Ar-H), 7.13 (t, J = 6.7 Hz, 1H, Ar-H), 6.87 (d, J = 8.5 Hz, 2H, Ar-H), 4.83 (s, 1H, NH), 1.88–1.41 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 157.37, 139.10, 129.22, 124.74, 122.99, 122.15, 115.05, 114.34, 112.87, 55.89, 40.05, 35.72, 29.00. HRMS (ESI) calcd for C23H26N3O [M + H]+ 360.2076, found 360.2048.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1292 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.13 (s, 1H, –OH), 8.55 (dd, J = 29.0, 6.8 Hz, 1H, Ar-H), 7.98 (dd, J = 13.1, 1.8 Hz, 1H, Ar-H), 7.82 (d, J = 8.3 Hz, 1H, Ar-H), 7.53 (d, J = 8.9 Hz, 1H, Ar-H), 7.37–7.28 (m, 1H, Ar-H), 7.09–6.93 (m, 2H, Ar-H), 4.77 (s, 1H, NH, Ar-H), 1.89–1.41 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 151.76, 149.38, 144.35, 139.90, 135.20, 124.54, 124.11, 122.43, 115.23, 112.13, 109.50, 55.91, 43.15, 35.74, 29.01. HRMS (ESI) calcd for C23H25FN3O [M + H]+ 378.1982, found 378.1950.
N), 1292 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.13 (s, 1H, –OH), 8.55 (dd, J = 29.0, 6.8 Hz, 1H, Ar-H), 7.98 (dd, J = 13.1, 1.8 Hz, 1H, Ar-H), 7.82 (d, J = 8.3 Hz, 1H, Ar-H), 7.53 (d, J = 8.9 Hz, 1H, Ar-H), 7.37–7.28 (m, 1H, Ar-H), 7.09–6.93 (m, 2H, Ar-H), 4.77 (s, 1H, NH, Ar-H), 1.89–1.41 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 151.76, 149.38, 144.35, 139.90, 135.20, 124.54, 124.11, 122.43, 115.23, 112.13, 109.50, 55.91, 43.15, 35.74, 29.01. HRMS (ESI) calcd for C23H25FN3O [M + H]+ 378.1982, found 378.1950.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1278 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 9.50 (s, 1H, –OH), 9.21 (s, 1H, –OH), 8.73 (d, J = 6.7 Hz, 1H, Ar-H), 7.77–7.60 (m, 2H, Ar-H), 7.55 (d, J = 2.1 Hz, 1H, Ar-H), 7.43 (dd, J = 8.2, 2.1 Hz, 1H, Ar-H), 7.30 (t, J = 6.3 Hz, 1H, Ar-H), 6.87 (d, J = 8.3 Hz, 1H, Ar-H), 4.92 (s, 1H, NH), 1.89–1.42 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) 146.46, 145.21, 138.64, 122.69, 119.67, 118.54, 115.60, 109.69, 109.38, 56.16, 42.86, 35.65, 29.00. HRMS (ESI) calcd for C23H26N3O2 [M + H]+ 376.2025, found 376.2001.
N), 1278 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 9.50 (s, 1H, –OH), 9.21 (s, 1H, –OH), 8.73 (d, J = 6.7 Hz, 1H, Ar-H), 7.77–7.60 (m, 2H, Ar-H), 7.55 (d, J = 2.1 Hz, 1H, Ar-H), 7.43 (dd, J = 8.2, 2.1 Hz, 1H, Ar-H), 7.30 (t, J = 6.3 Hz, 1H, Ar-H), 6.87 (d, J = 8.3 Hz, 1H, Ar-H), 4.92 (s, 1H, NH), 1.89–1.42 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) 146.46, 145.21, 138.64, 122.69, 119.67, 118.54, 115.60, 109.69, 109.38, 56.16, 42.86, 35.65, 29.00. HRMS (ESI) calcd for C23H26N3O2 [M + H]+ 376.2025, found 376.2001.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1278 (C–N), 1126 (O–CH2–O). 1H NMR (400 MHz, CDCl3) δ 8.25 (d, J = 6.9 Hz, 1H, Ar-H), 7.50 (t, J = 5.4 Hz, 2H, Ar-H), 7.43 (dd, J = 8.4, 2.0 Hz, 1H, Ar-H), 7.28 (s, 1H, Ar-H), 7.10 (ddd, J = 8.9, 6.6, 1.2 Hz, 1H, Ar-H), 6.91 (d, J = 8.4 Hz, 1H, Ar-H), 6.74 (td, J = 6.8, 1.0, 1H, Ar-H), 4.30 (s, 4H, –CH2), 3.03 (s, 1H, NH), 1.94–1.46 (m, 15H, adamantyl-H). 13C NMR (100 MHz, CDCl3) δ 143.33, 143.01, 141.76, 138.97, 128.69, 121.46, 117.01, 111.15, 64.50, 65.37, 56.62, 43.81, 36.16, 29.67. HRMS (ESI) calcd for C25H28N3O2 [M + H]+ 424.20, found 402.2149.
N), 1278 (C–N), 1126 (O–CH2–O). 1H NMR (400 MHz, CDCl3) δ 8.25 (d, J = 6.9 Hz, 1H, Ar-H), 7.50 (t, J = 5.4 Hz, 2H, Ar-H), 7.43 (dd, J = 8.4, 2.0 Hz, 1H, Ar-H), 7.28 (s, 1H, Ar-H), 7.10 (ddd, J = 8.9, 6.6, 1.2 Hz, 1H, Ar-H), 6.91 (d, J = 8.4 Hz, 1H, Ar-H), 6.74 (td, J = 6.8, 1.0, 1H, Ar-H), 4.30 (s, 4H, –CH2), 3.03 (s, 1H, NH), 1.94–1.46 (m, 15H, adamantyl-H). 13C NMR (100 MHz, CDCl3) δ 143.33, 143.01, 141.76, 138.97, 128.69, 121.46, 117.01, 111.15, 64.50, 65.37, 56.62, 43.81, 36.16, 29.67. HRMS (ESI) calcd for C25H28N3O2 [M + H]+ 424.20, found 402.2149.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1282 (C–N), 1186 (O–CH2). 1H NMR (400 MHz, DMSO-d6) δ 9.36 (s, 1H, –OH), 7.80–7.72 (m, 2H, Ar-H), 7.61 (d, J = 6.4 Hz, 1H, Ar-H), 7.22 (d, J = 5.7 Hz, 1H, Ar-H), 6.90 (d, J = 8.3 Hz, 1H, Ar-H), 4.97 (s, 1H, NH), 4.12 (q, J = 7.0 Hz, 2H, –CH2–), 1.89–1.36 (m, 18H, adamantyl-H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 147.34, 146.35, 138.59, 126.03, 120.98, 112.78, 109.32, 63.95, 56.10, 40.08, 35.69, 29.02, 14.83. HRMS (ESI) calcd for C25H29ClN3O2 [M + H]+ 438.1938, found 438.1916.
N), 1282 (C–N), 1186 (O–CH2). 1H NMR (400 MHz, DMSO-d6) δ 9.36 (s, 1H, –OH), 7.80–7.72 (m, 2H, Ar-H), 7.61 (d, J = 6.4 Hz, 1H, Ar-H), 7.22 (d, J = 5.7 Hz, 1H, Ar-H), 6.90 (d, J = 8.3 Hz, 1H, Ar-H), 4.97 (s, 1H, NH), 4.12 (q, J = 7.0 Hz, 2H, –CH2–), 1.89–1.36 (m, 18H, adamantyl-H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 147.34, 146.35, 138.59, 126.03, 120.98, 112.78, 109.32, 63.95, 56.10, 40.08, 35.69, 29.02, 14.83. HRMS (ESI) calcd for C25H29ClN3O2 [M + H]+ 438.1938, found 438.1916.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1269 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 9.62 (s, 1H, –OH), 8.47 (d, J = 7.3 Hz, 1H, Ar-H), 7.99 (d, J = 8.7 Hz, 2H, Ar-H), 6.99 (dd, J = 7.3, 2.0 Hz, 1H, Ar-H), 6.82 (d, J = 8.7 Hz, 2H, Ar-H), 6.82 (d, J = 8.7 Hz, 1H, Ar-H), 4.69 (s, 1H, NH), 1.87–1.40 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 157.00, 139.83, 137.65, 129.77, 129.06, 122.32, 115.89, 114.87, 114.16, 112.62, 55.87, 43.13, 35.75, 29.02. HRMS (ESI) calcd for C23H25ClN3O [M + H]+ 394.1686, found 394.1656.
N), 1269 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 9.62 (s, 1H, –OH), 8.47 (d, J = 7.3 Hz, 1H, Ar-H), 7.99 (d, J = 8.7 Hz, 2H, Ar-H), 6.99 (dd, J = 7.3, 2.0 Hz, 1H, Ar-H), 6.82 (d, J = 8.7 Hz, 2H, Ar-H), 6.82 (d, J = 8.7 Hz, 1H, Ar-H), 4.69 (s, 1H, NH), 1.87–1.40 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 157.00, 139.83, 137.65, 129.77, 129.06, 122.32, 115.89, 114.87, 114.16, 112.62, 55.87, 43.13, 35.75, 29.02. HRMS (ESI) calcd for C23H25ClN3O [M + H]+ 394.1686, found 394.1656.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1280 (C–N), 1184 (O–CH2). 1H NMR (400 MHz, DMSO-d6) δ 9.25 (s, 1H, –OH), 8.51 (d, J = 7.3 Hz, 1H, Ar-H), 7.92–7.67 (m, 2H, Ar-H), 7.60 (d, J = 6.6 Hz, 1H, Ar-H), 7.22 (d, J = 7.1 Hz, 1H, Ar-H), 6.88 (d, J = 8.3 Hz, 1H, Ar-H), 4.86 (s, 1H, NH), 4.12 (q, J = 6.9 Hz, 2H, –CH2–), 1.89–1.36 (m, 18H, adamantyl-H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 147.06, 146.27, 139.38, 134.79, 125.64, 123.21, 122.58, 120.94, 116.35, 116.10, 115.37, 113.17, 63.91, 56.02, 40.09, 35.71, 29.02, 14.85. HRMS (ESI) calcd for C25H29BrN3O2 [M + H]+ 482.1443, found 482.1408.
N), 1280 (C–N), 1184 (O–CH2). 1H NMR (400 MHz, DMSO-d6) δ 9.25 (s, 1H, –OH), 8.51 (d, J = 7.3 Hz, 1H, Ar-H), 7.92–7.67 (m, 2H, Ar-H), 7.60 (d, J = 6.6 Hz, 1H, Ar-H), 7.22 (d, J = 7.1 Hz, 1H, Ar-H), 6.88 (d, J = 8.3 Hz, 1H, Ar-H), 4.86 (s, 1H, NH), 4.12 (q, J = 6.9 Hz, 2H, –CH2–), 1.89–1.36 (m, 18H, adamantyl-H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 147.06, 146.27, 139.38, 134.79, 125.64, 123.21, 122.58, 120.94, 116.35, 116.10, 115.37, 113.17, 63.91, 56.02, 40.09, 35.71, 29.02, 14.85. HRMS (ESI) calcd for C25H29BrN3O2 [M + H]+ 482.1443, found 482.1408.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1253 (C–N), 1163 (O–CH2). 1H NMR (400 MHz, DMSO-d6) δ 9.07 (s, 1H, –OH), 8.59 (d, J = 7.2 Hz, 1H, Ar-H), 7.99–7.74 (m, 2H, Ar-H), 7.67 (d, J = 6.5 Hz, 1H, Ar-H), 7.10 (d, J = 5.6 Hz, 1H, Ar-H), 6.85 (d, J = 8.2 Hz, 1H, Ar-H), 4.78 (s, 1H, NH), 4.11 (q, J = 6.9 Hz, 2H, –CH2–), 1.89–1.37 (m, 18H, adamantyl-H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 146.57, 146.12, 141.08, 138.59, 123.58, 123.31, 122.51, 120.92, 113.20, 109.50, 106.21, 63.83, 56.08, 43.28, 35.75, 29.02, 14.87. HRMS (ESI) calcd for C26H29F3N3O2 [M + H]+ 472.2212, found 472.2277.
N), 1253 (C–N), 1163 (O–CH2). 1H NMR (400 MHz, DMSO-d6) δ 9.07 (s, 1H, –OH), 8.59 (d, J = 7.2 Hz, 1H, Ar-H), 7.99–7.74 (m, 2H, Ar-H), 7.67 (d, J = 6.5 Hz, 1H, Ar-H), 7.10 (d, J = 5.6 Hz, 1H, Ar-H), 6.85 (d, J = 8.2 Hz, 1H, Ar-H), 4.78 (s, 1H, NH), 4.11 (q, J = 6.9 Hz, 2H, –CH2–), 1.89–1.37 (m, 18H, adamantyl-H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 146.57, 146.12, 141.08, 138.59, 123.58, 123.31, 122.51, 120.92, 113.20, 109.50, 106.21, 63.83, 56.08, 43.28, 35.75, 29.02, 14.87. HRMS (ESI) calcd for C26H29F3N3O2 [M + H]+ 472.2212, found 472.2277.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1253 (C–N), 1132 (O–CH2). 1H NMR (400 MHz, DMSO-d6) δ 9.58 (s, 1H, –OH), 8.88 (d, J = 7.1 Hz, 1H, Ar-H), 7.79 (d, J = 1.7 Hz, 1H, Ar-H), 7.66 (d, J = 6.6 Hz, 1H, Ar-H), 7.57 (d, J = 6.9 Hz, 1H, Ar-H), 7.50–7.18 (m, 2H, Ar-H), 6.96 (d, J = 8.3 Hz, 1H, Ar-H), 5.27 (s, 1H, NH), 4.15 (q, J = 6.9 Hz, 2H, –CH2–), 1.89–1.37 (m, 18H, adamantyl-H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 147.26, 145.43, 134.77, 125.17, 123.55, 120.31, 117.48, 116.93, 115.86, 114.50, 112.14, 62.90, 55.68, 38.91, 34.42, 27.87, 13.63. HRMS (ESI) calcd for C26H29N4O2 [M + H]+ 429.2291, found 429.2217.
N), 1253 (C–N), 1132 (O–CH2). 1H NMR (400 MHz, DMSO-d6) δ 9.58 (s, 1H, –OH), 8.88 (d, J = 7.1 Hz, 1H, Ar-H), 7.79 (d, J = 1.7 Hz, 1H, Ar-H), 7.66 (d, J = 6.6 Hz, 1H, Ar-H), 7.57 (d, J = 6.9 Hz, 1H, Ar-H), 7.50–7.18 (m, 2H, Ar-H), 6.96 (d, J = 8.3 Hz, 1H, Ar-H), 5.27 (s, 1H, NH), 4.15 (q, J = 6.9 Hz, 2H, –CH2–), 1.89–1.37 (m, 18H, adamantyl-H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 147.26, 145.43, 134.77, 125.17, 123.55, 120.31, 117.48, 116.93, 115.86, 114.50, 112.14, 62.90, 55.68, 38.91, 34.42, 27.87, 13.63. HRMS (ESI) calcd for C26H29N4O2 [M + H]+ 429.2291, found 429.2217.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1282 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 9.79 (s, 1H, –OH), 8.52 (d, J = 7.3 Hz, 1H, Ar-H), 7.98 (d, J = 8.6 Hz, 2H, Ar-H), 7.86 (s, 1H, Ar-H), 7.25 (d, J = 5.8 Hz, 1H, Ar-H), 6.86 (d, J = 8.6 Hz, 2H, Ar-H), 4.84 (s, 1H, NH), 1.87–1.40 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 157.56, 139.23, 129.23, 125.74, 122.61, 116.34, 115.10, 108.78, 56.04, 40.09, 35.69, 29.01. HRMS (ESI) calcd for C23H25BrN3O [M + H]+ 438.1181, found 438.1148.
N), 1282 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 9.79 (s, 1H, –OH), 8.52 (d, J = 7.3 Hz, 1H, Ar-H), 7.98 (d, J = 8.6 Hz, 2H, Ar-H), 7.86 (s, 1H, Ar-H), 7.25 (d, J = 5.8 Hz, 1H, Ar-H), 6.86 (d, J = 8.6 Hz, 2H, Ar-H), 4.84 (s, 1H, NH), 1.87–1.40 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 157.56, 139.23, 129.23, 125.74, 122.61, 116.34, 115.10, 108.78, 56.04, 40.09, 35.69, 29.01. HRMS (ESI) calcd for C23H25BrN3O [M + H]+ 438.1181, found 438.1148.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1267 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.69 (s, 1H, –OH), 8.30 (d, J = 7.0 Hz, 1H, Ar-H), 7.82 (d, J = 8.9 Hz, 1H, Ar-H), 7.22–7.20 (m, 2H, Ar-H), 6.89–6.76 (m, 2H, Ar-H), 4.27 (s, 1H, NH), 2.64 (q, J = 7.5 Hz, 2H, –CH2–), 1.84–1.36 (m, 15H, adamantyl-H), 1.25–1.21 (m, 3H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.60, 141.44, 141.40, 140.31, 132.75, 126.72, 123.19, 118.18, 114.22, 113.61, 112.68, 55.10, 40.09, 35.76, 28.96, 27.57, 14.48. HRMS (ESI) calcd for C25H29N4O3 [M + H]+ 433.2240, found 433.2207.
N), 1267 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.69 (s, 1H, –OH), 8.30 (d, J = 7.0 Hz, 1H, Ar-H), 7.82 (d, J = 8.9 Hz, 1H, Ar-H), 7.22–7.20 (m, 2H, Ar-H), 6.89–6.76 (m, 2H, Ar-H), 4.27 (s, 1H, NH), 2.64 (q, J = 7.5 Hz, 2H, –CH2–), 1.84–1.36 (m, 15H, adamantyl-H), 1.25–1.21 (m, 3H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 160.60, 141.44, 141.40, 140.31, 132.75, 126.72, 123.19, 118.18, 114.22, 113.61, 112.68, 55.10, 40.09, 35.76, 28.96, 27.57, 14.48. HRMS (ESI) calcd for C25H29N4O3 [M + H]+ 433.2240, found 433.2207.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1271 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.74 (s, 1H, –OH), 8.37 (d, J = 6.9 Hz, 1H, Ar-H), 7.86 (d, J = 8.9, 2H, Ar-H), 7.78 (d, J = 1.4 Hz, 1H, Ar-H), 7.21 (d, J = 2.7 Hz, 1H, Ar-H), 7.07 (dd, J = 7.3, 1.9 Hz, 1H, Ar-H), 6.89 (dd, J = 8.9, 2.7 Hz, 1H, Ar-H), 4.48 (s, 1H, NH), 1.84–1.36 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 160.77, 141.30, 140.96, 136.15, 132.09, 124.31, 118.64, 118.26, 116.85, 114.75, 114.61, 55.31, 40.09, 35.70, 28.96. HRMS (ESI) calcd for C23H24BrN4O3 [M + H]+ 483.1032, found 483.1001.
N), 1271 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.74 (s, 1H, –OH), 8.37 (d, J = 6.9 Hz, 1H, Ar-H), 7.86 (d, J = 8.9, 2H, Ar-H), 7.78 (d, J = 1.4 Hz, 1H, Ar-H), 7.21 (d, J = 2.7 Hz, 1H, Ar-H), 7.07 (dd, J = 7.3, 1.9 Hz, 1H, Ar-H), 6.89 (dd, J = 8.9, 2.7 Hz, 1H, Ar-H), 4.48 (s, 1H, NH), 1.84–1.36 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 160.77, 141.30, 140.96, 136.15, 132.09, 124.31, 118.64, 118.26, 116.85, 114.75, 114.61, 55.31, 40.09, 35.70, 28.96. HRMS (ESI) calcd for C23H24BrN4O3 [M + H]+ 483.1032, found 483.1001.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1230 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.81 (s, 1H, –OH), 8.61 (d, J = 7.2 Hz, 1H, Ar-H), 8.05–7.82 (m, 2H, Ar-H), 7.34–7.10 (m, 2H, Ar-H), 6.93 (dd, J = 8.9, 2.6 Hz, 1H, Ar-H), 4.65 (s, 1H, NH), 1.85–1.36 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 160.92, 141.24, 138.79, 138.11, 131.83, 125.59, 125.52, 118.34, 114.88, 106.77, 55.56, 40.09, 35.66, 28.95. HRMS (ESI) calcd for C24H24F3N4O3 [M + H]+ 473.1801, found 473.1806.
N), 1230 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.81 (s, 1H, –OH), 8.61 (d, J = 7.2 Hz, 1H, Ar-H), 8.05–7.82 (m, 2H, Ar-H), 7.34–7.10 (m, 2H, Ar-H), 6.93 (dd, J = 8.9, 2.6 Hz, 1H, Ar-H), 4.65 (s, 1H, NH), 1.85–1.36 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 160.92, 141.24, 138.79, 138.11, 131.83, 125.59, 125.52, 118.34, 114.88, 106.77, 55.56, 40.09, 35.66, 28.95. HRMS (ESI) calcd for C24H24F3N4O3 [M + H]+ 473.1801, found 473.1806.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1234 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.28 (s, 1H, –OH), 8.55 (d, J = 7.2 Hz, 1H, Ar-H), 8.24 (s, 1H, Ar-H), 7.90 (d, J = 8.9 Hz, 1H, Ar-H), 7.32–7.13 (m, 2H, Ar-H), 6.93 (dd, J = 8.9, 2.5 Hz, 1H, Ar-H), 5.32 (s, 1H, NH), 1.84–1.36 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 160.99, 141.16, 138.96, 138.82, 131.58, 126.39, 123.45, 118.21, 115.04, 111.48, 104.90, 55.74, 40.08, 35.63, 28.95. HRMS (ESI) calcd for C24H24N5O3 [M + H]+ 430.1879, found 430.1847.
N), 1234 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.28 (s, 1H, –OH), 8.55 (d, J = 7.2 Hz, 1H, Ar-H), 8.24 (s, 1H, Ar-H), 7.90 (d, J = 8.9 Hz, 1H, Ar-H), 7.32–7.13 (m, 2H, Ar-H), 6.93 (dd, J = 8.9, 2.5 Hz, 1H, Ar-H), 5.32 (s, 1H, NH), 1.84–1.36 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 160.99, 141.16, 138.96, 138.82, 131.58, 126.39, 123.45, 118.21, 115.04, 111.48, 104.90, 55.74, 40.08, 35.63, 28.95. HRMS (ESI) calcd for C24H24N5O3 [M + H]+ 430.1879, found 430.1847.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1269 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 8.42 (d, J = 6.9 Hz, 1H, Ar-H), 8.19 (d, J = 8.6 Hz, 2H, Ar-H), 7.80–7.53 (m, 2H, Ar-H), 7.45 (d, J = 9.0 Hz, 1H, Ar-H), 7.18 (ddd, J = 9.0, 6.6, 1.2 Hz, 1H, Ar-H), 6.88 (td, J = 6.8, 1.0 Hz, 1H, Ar-H), 4.69 (s, 1H, NH), 1.88–1.41 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 141.11, 136.71, 134.68, 130.81, 129.54, 124.30, 124.17, 123.17, 119.97, 116.60, 111.12, 109.50, 55.96, 43.24, 35.77, 29.02. HRMS (ESI) calcd for C23H25BrN3 [M + H]+ 422.1232, found 422.1200.
N), 1269 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 8.42 (d, J = 6.9 Hz, 1H, Ar-H), 8.19 (d, J = 8.6 Hz, 2H, Ar-H), 7.80–7.53 (m, 2H, Ar-H), 7.45 (d, J = 9.0 Hz, 1H, Ar-H), 7.18 (ddd, J = 9.0, 6.6, 1.2 Hz, 1H, Ar-H), 6.88 (td, J = 6.8, 1.0 Hz, 1H, Ar-H), 4.69 (s, 1H, NH), 1.88–1.41 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 141.11, 136.71, 134.68, 130.81, 129.54, 124.30, 124.17, 123.17, 119.97, 116.60, 111.12, 109.50, 55.96, 43.24, 35.77, 29.02. HRMS (ESI) calcd for C23H25BrN3 [M + H]+ 422.1232, found 422.1200.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1282 (C–N), 1122 (O–CH2CH3). 1H NMR (400 MHz, DMSO-d6) δ 9.34 (s, 1H, –OH), 8.67 (d, J = 6.8 Hz, 1H, Ar-H), 7.77 (d, J = 1.9 Hz, 1H, Ar-H), 7.67 (d, J = 8.9 Hz, 1H, Ar-H), 7.61 (dd, J = 8.3, 1.9 Hz, 1H, Ar-H), 7.55 (t, J = 7.7 Hz, 1H, Ar-H), 7.18 (t, J = 6.7 Hz, 1H, Ar-H), 6.91 (d, J = 8.3 Hz, 1H, Ar-H), 4.93 (s, 1H, NH), 4.16 (q, J = 6.9 Hz, 2H, –CH2–), 1.89–1.37 (m, 18H, adamantyl-H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 147.43, 146.40, 138.22, 136.68, 136.44, 125.13, 122.37, 121.56, 121.04, 115.48, 114.48, 113.26, 63.97, 56.03, 40.08, 35.68, 29.01, 14.82. HRMS (ESI) calcd for C25H30N3O2 [M + H]+ 404.2338, found 404.2314.
N), 1282 (C–N), 1122 (O–CH2CH3). 1H NMR (400 MHz, DMSO-d6) δ 9.34 (s, 1H, –OH), 8.67 (d, J = 6.8 Hz, 1H, Ar-H), 7.77 (d, J = 1.9 Hz, 1H, Ar-H), 7.67 (d, J = 8.9 Hz, 1H, Ar-H), 7.61 (dd, J = 8.3, 1.9 Hz, 1H, Ar-H), 7.55 (t, J = 7.7 Hz, 1H, Ar-H), 7.18 (t, J = 6.7 Hz, 1H, Ar-H), 6.91 (d, J = 8.3 Hz, 1H, Ar-H), 4.93 (s, 1H, NH), 4.16 (q, J = 6.9 Hz, 2H, –CH2–), 1.89–1.37 (m, 18H, adamantyl-H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 147.43, 146.40, 138.22, 136.68, 136.44, 125.13, 122.37, 121.56, 121.04, 115.48, 114.48, 113.26, 63.97, 56.03, 40.08, 35.68, 29.01, 14.82. HRMS (ESI) calcd for C25H30N3O2 [M + H]+ 404.2338, found 404.2314.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1294 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.54 (s, 1H, –OH), 8.52 (d, J = 6.1, 1H, Ar-H), 8.38 (d, J = 1.5, 1H, Ar-H), 8.03 (dd, J = 8.5, 1.7 Hz, 1H, Ar-H), 7.57–7.49 (m, 1H, Ar-H), 7.40–7.28 (m, 1H, Ar-H), 7.03 (dd, J = 15.0, 7.6, 2H, Ar-H), 4.79 (s, 1H, NH), 1.90–1.42 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 153.52, 139.94, 134.55, 131.85, 127.96, 125.87, 124.40, 122.40, 115.91, 115.25, 112.13, 108.96, 55.89, 43.24, 35.75, 29.02. HRMS (ESI) calcd for C23H25BrN3O [M + H]+ 438.1181, found 438.1149.
N), 1294 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.54 (s, 1H, –OH), 8.52 (d, J = 6.1, 1H, Ar-H), 8.38 (d, J = 1.5, 1H, Ar-H), 8.03 (dd, J = 8.5, 1.7 Hz, 1H, Ar-H), 7.57–7.49 (m, 1H, Ar-H), 7.40–7.28 (m, 1H, Ar-H), 7.03 (dd, J = 15.0, 7.6, 2H, Ar-H), 4.79 (s, 1H, NH), 1.90–1.42 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 153.52, 139.94, 134.55, 131.85, 127.96, 125.87, 124.40, 122.40, 115.91, 115.25, 112.13, 108.96, 55.89, 43.24, 35.75, 29.02. HRMS (ESI) calcd for C23H25BrN3O [M + H]+ 438.1181, found 438.1149.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1278 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.26 (s, 1H, –OH), 8.55 (d, J = 6.9 Hz, 1H, Ar-H), 7.82–7.41 (m, 2H, Ar-H), 7.46–7.13 (m, 1H, Ar-H), 7.03 (t, J = 6.7 Hz, 1H, Ar-H), 6.83–6.56 (m, 2H, Ar-H), 4.08 (s, 1H, NH), 1.85–1.38 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 161.13, 159.05, 140.01, 132.22, 126.00, 124.37, 123.61, 115.26, 112.42, 111.87, 102.85, 102.61, 55.24, 40.07, 35.66, 28.92. HRMS (ESI) calcd for C23H25FN3O [M + H]+ 378.1982, found 378.1950.
N), 1278 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.26 (s, 1H, –OH), 8.55 (d, J = 6.9 Hz, 1H, Ar-H), 7.82–7.41 (m, 2H, Ar-H), 7.46–7.13 (m, 1H, Ar-H), 7.03 (t, J = 6.7 Hz, 1H, Ar-H), 6.83–6.56 (m, 2H, Ar-H), 4.08 (s, 1H, NH), 1.85–1.38 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 161.13, 159.05, 140.01, 132.22, 126.00, 124.37, 123.61, 115.26, 112.42, 111.87, 102.85, 102.61, 55.24, 40.07, 35.66, 28.92. HRMS (ESI) calcd for C23H25FN3O [M + H]+ 378.1982, found 378.1950.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1296 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.47 (s, 1H, –OH), 8.52 (d, J = 6.8 Hz, 1H, Ar-H), 8.21 (d, J = 2.0 Hz, 1H, Ar-H), 7.99 (dd, J = 8.5, 2.0 Hz, 1H, Ar-H), 7.53 (d, J = 8.9 Hz, 1H, Ar-H), 7.43–7.25 (m, 1H, Ar-H), 7.17–6.85 (m, 2H, Ar-H), 4.79 (s, 1H, NH), 1.89–1.41 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 152.66, 139.72, 138.32, 128.93, 127.40, 126.33, 125.25, 124.63, 122.48, 119.38, 116.28, 115.00, 112.40, 55.92, 43.19, 35.74, 29.02. HRMS (ESI) calcd for C23H25ClN3O [M + H]+ 394.1686, found 394.1657.
N), 1296 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.47 (s, 1H, –OH), 8.52 (d, J = 6.8 Hz, 1H, Ar-H), 8.21 (d, J = 2.0 Hz, 1H, Ar-H), 7.99 (dd, J = 8.5, 2.0 Hz, 1H, Ar-H), 7.53 (d, J = 8.9 Hz, 1H, Ar-H), 7.43–7.25 (m, 1H, Ar-H), 7.17–6.85 (m, 2H, Ar-H), 4.79 (s, 1H, NH), 1.89–1.41 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 152.66, 139.72, 138.32, 128.93, 127.40, 126.33, 125.25, 124.63, 122.48, 119.38, 116.28, 115.00, 112.40, 55.92, 43.19, 35.74, 29.02. HRMS (ESI) calcd for C23H25ClN3O [M + H]+ 394.1686, found 394.1657.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1276 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 9.34 (s, 1H, –OH), 8.38 (d, J = 6.2 Hz, 1H, Ar-H), 8.06–7.70 (m, 2H, Ar-H), 7.40 (d, J = 8.71 Hz, 1H, Ar-H), 7.21–7.01 (m, 1H, Ar-H), 6.81 (dd, J = 15.6, 7.2 Hz, 2H, Ar-H), 4.51 (s, 1H, NH), 2.19 (s, 3H, –CH3), 1.89–1.42 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 154.50, 140.73, 138.51, 130.15, 126.18, 123.91, 123.28, 122.98, 121.60, 116.13, 113.98, 110.58, 55.68, 43.33, 35.86, 29.04, 16.09. HRMS (ESI) calcd for C24H28N3O [M + H]+ 374.2232, found 374.2204.
N), 1276 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 9.34 (s, 1H, –OH), 8.38 (d, J = 6.2 Hz, 1H, Ar-H), 8.06–7.70 (m, 2H, Ar-H), 7.40 (d, J = 8.71 Hz, 1H, Ar-H), 7.21–7.01 (m, 1H, Ar-H), 6.81 (dd, J = 15.6, 7.2 Hz, 2H, Ar-H), 4.51 (s, 1H, NH), 2.19 (s, 3H, –CH3), 1.89–1.42 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 154.50, 140.73, 138.51, 130.15, 126.18, 123.91, 123.28, 122.98, 121.60, 116.13, 113.98, 110.58, 55.68, 43.33, 35.86, 29.04, 16.09. HRMS (ESI) calcd for C24H28N3O [M + H]+ 374.2232, found 374.2204.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1276 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.78 (s, 1H, –OH), 8.29 (d, J = 6.9 Hz, 1H, Ar-H), 7.86 (d, J = 8.9 Hz, 1H, Ar-H), 7.45 (d, J = 9.1 Hz, 1H, Ar-H), 7.25–7.09 (m, 2H, Ar-H), 6.99–6.78 (m, 2H, Ar-H), 4.59 (d, J = 5.4 Hz, 1H, NH), 2.11–0.85 (m, 11H, cyclohexyl-H). 13C NMR (100 MHz, DMSO-d6) δ 160.72, 141.20, 140.31, 132.20, 131.74, 126.83, 126.30, 123.57, 123.38, 117.84, 116.92, 114.42, 111.47, 109.50, 55.72, 33.03, 25.33, 24.08. HRMS (ESI) calcd for C19H21N4O3 [M + H]+ 353.1614, found 353.1611.
N), 1276 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.78 (s, 1H, –OH), 8.29 (d, J = 6.9 Hz, 1H, Ar-H), 7.86 (d, J = 8.9 Hz, 1H, Ar-H), 7.45 (d, J = 9.1 Hz, 1H, Ar-H), 7.25–7.09 (m, 2H, Ar-H), 6.99–6.78 (m, 2H, Ar-H), 4.59 (d, J = 5.4 Hz, 1H, NH), 2.11–0.85 (m, 11H, cyclohexyl-H). 13C NMR (100 MHz, DMSO-d6) δ 160.72, 141.20, 140.31, 132.20, 131.74, 126.83, 126.30, 123.57, 123.38, 117.84, 116.92, 114.42, 111.47, 109.50, 55.72, 33.03, 25.33, 24.08. HRMS (ESI) calcd for C19H21N4O3 [M + H]+ 353.1614, found 353.1611.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1261 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.77 (s, 1H, –OH), 8.41 (d, J = 6.9 Hz, 2H, Ar-H), 7.88 (d, J = 8.9 Hz, 1H, Ar-H), 7.44 (d, J = 9.0 Hz, 1H, Ar-H), 7.25–7.05 (m, 2H, Ar-H), 6.99–6.78 (m, 2H, Ar-H), 4.20 (s, 2H, NH), 0.94 (s, 6H, –CH3), 0.89 (s, 9H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 160, 141.22, 140.94, 135.86, 133.05, 126.84, 124.35, 124.18, 123.92, 118.59, 116.79, 114.45, 111.24, 59.35, 55.38, 31.48, 30.98, 28.46. HRMS (ESI) calcd for C20H25N4O3 [M + H]+ 369.1848, found 369.1845.
N), 1261 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.77 (s, 1H, –OH), 8.41 (d, J = 6.9 Hz, 2H, Ar-H), 7.88 (d, J = 8.9 Hz, 1H, Ar-H), 7.44 (d, J = 9.0 Hz, 1H, Ar-H), 7.25–7.05 (m, 2H, Ar-H), 6.99–6.78 (m, 2H, Ar-H), 4.20 (s, 2H, NH), 0.94 (s, 6H, –CH3), 0.89 (s, 9H, –CH3). 13C NMR (100 MHz, DMSO-d6) δ 160, 141.22, 140.94, 135.86, 133.05, 126.84, 124.35, 124.18, 123.92, 118.59, 116.79, 114.45, 111.24, 59.35, 55.38, 31.48, 30.98, 28.46. HRMS (ESI) calcd for C20H25N4O3 [M + H]+ 369.1848, found 369.1845.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1230 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 12.06 (s, 1H, NH), 8.93 (d, J = 6.8 Hz, 1H, Ar-H), 8.25 (d, J = 2.0 Hz, 1H, Ar-H), 8.13 (dd, J = 8.8, 5.4 Hz, 1H, Ar-H), 7.95–7.79 (m, 2H, Ar-H), 7.49 (dd, J = 10.0, 4.5 Hz, 1H, Ar-H), 7.32 (dd, J = 9.8, 2.3 Hz, 1H, Ar-H), 7.05 (td, J = 9.4, 2.3 Hz, 1H, Ar-H), 5.27 (s, 1H, NH), 1.82–1.34 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) 156.05, 136.85, 132.97, 132.13, 129.30, 125.87, 124.90, 122.41, 116.12, 113.25, 111.46, 110.50, 110.24, 106.05, 105.80, 102.69, 56.11, 40.06, 35.54, 28.88. HRMS (ESI) calcd for C25H26FN4 [M + H]+ 401.2142, found 401.2114.
N), 1230 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 12.06 (s, 1H, NH), 8.93 (d, J = 6.8 Hz, 1H, Ar-H), 8.25 (d, J = 2.0 Hz, 1H, Ar-H), 8.13 (dd, J = 8.8, 5.4 Hz, 1H, Ar-H), 7.95–7.79 (m, 2H, Ar-H), 7.49 (dd, J = 10.0, 4.5 Hz, 1H, Ar-H), 7.32 (dd, J = 9.8, 2.3 Hz, 1H, Ar-H), 7.05 (td, J = 9.4, 2.3 Hz, 1H, Ar-H), 5.27 (s, 1H, NH), 1.82–1.34 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) 156.05, 136.85, 132.97, 132.13, 129.30, 125.87, 124.90, 122.41, 116.12, 113.25, 111.46, 110.50, 110.24, 106.05, 105.80, 102.69, 56.11, 40.06, 35.54, 28.88. HRMS (ESI) calcd for C25H26FN4 [M + H]+ 401.2142, found 401.2114.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1261 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 11.89 (d, J = 1.7 Hz, 1H, NH), 8.93 (d, J = 6.8 Hz, 1H, Ar-H), 8.24 (d, J = 2.5 Hz, 1H, Ar-H), 7.98 (d, J = 8.8 Hz, 1H, Ar-H), 7.91–7.82 (m, 1H, Ar-H), 7.69 (d, J = 2.1 Hz, 1H, Ar-H), 7.53–7.37 (m, 2H, Ar-H), 6.86 (dd, J = 8.8, 2.3 Hz, 1H, Ar-H), 5.34 (s, 1H, NH), 3.86 (s, 3H, –OCH3), 1.82–1.35 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 153.86, 136.79, 131.25, 127.89, 122.08, 116.06, 112.69, 111.41, 102.73, 102.09, 56.03, 55.44, 40.11, 35.58, 28.91. HRMS (ESI) calcd for C26H29N4O [M + H]+ 413.2341, found 413.2314.
N), 1261 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 11.89 (d, J = 1.7 Hz, 1H, NH), 8.93 (d, J = 6.8 Hz, 1H, Ar-H), 8.24 (d, J = 2.5 Hz, 1H, Ar-H), 7.98 (d, J = 8.8 Hz, 1H, Ar-H), 7.91–7.82 (m, 1H, Ar-H), 7.69 (d, J = 2.1 Hz, 1H, Ar-H), 7.53–7.37 (m, 2H, Ar-H), 6.86 (dd, J = 8.8, 2.3 Hz, 1H, Ar-H), 5.34 (s, 1H, NH), 3.86 (s, 3H, –OCH3), 1.82–1.35 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 153.86, 136.79, 131.25, 127.89, 122.08, 116.06, 112.69, 111.41, 102.73, 102.09, 56.03, 55.44, 40.11, 35.58, 28.91. HRMS (ESI) calcd for C26H29N4O [M + H]+ 413.2341, found 413.2314.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1259 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 12.00 (d, J = 2.0 Hz, 1H, NH), 8.94 (d, J = 6.8 Hz, 1H, Ar-H), 8.28 (d, J = 2.7 Hz, 1H, Ar-H), 8.12 (d, J = 7.8 Hz, 1H, Ar-H), 7.96–7.85 (m, 2H, Ar-H), 7.51 (ddd, J = 9.5, 8.1, 4.7 Hz, 2H, Ar-H), 7.29–7.11 (m, 2H, Ar-H), 5.27 (s, 1H, NH), 1.82–1.34 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 136.78, 136.22, 132.14, 127.55, 122.16, 119.89, 116.21, 112.13, 111.45, 102.14, 56.01, 40.06, 35.55, 28.88. HRMS (ESI) calcd for C25H27N4 [M + H]+ 383.2236, found 383.2209.
N), 1259 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 12.00 (d, J = 2.0 Hz, 1H, NH), 8.94 (d, J = 6.8 Hz, 1H, Ar-H), 8.28 (d, J = 2.7 Hz, 1H, Ar-H), 8.12 (d, J = 7.8 Hz, 1H, Ar-H), 7.96–7.85 (m, 2H, Ar-H), 7.51 (ddd, J = 9.5, 8.1, 4.7 Hz, 2H, Ar-H), 7.29–7.11 (m, 2H, Ar-H), 5.27 (s, 1H, NH), 1.82–1.34 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 136.78, 136.22, 132.14, 127.55, 122.16, 119.89, 116.21, 112.13, 111.45, 102.14, 56.01, 40.06, 35.55, 28.88. HRMS (ESI) calcd for C25H27N4 [M + H]+ 383.2236, found 383.2209.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1257 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 11.89 (s, 1H, NH), 8.92 (d, J = 6.3 Hz, 1H, Ar-H), 8.26 (s, 1H, Ar-H), 7.91 (dd, J = 24.9, 11.3 Hz, 3H, Ar-H), 7.58–7.30 (m, 2H, Ar-H), 7.06 (d, J = 8.1 Hz, 1H, Ar-H), 5.28 (s, 1H, NH), 2.45 (s, 3H, –CH3), 1.83–1.36 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 136.85, 134.56, 131.75, 128.50, 123.67, 120.15, 116.05, 111.75, 111.55, 101.72, 56.03, 40.07, 35.58, 28.90, 21.33. HRMS (ESI) calcd for C26H29N4 [M + H]+ 397.2392, found 397.2366.
N), 1257 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 11.89 (s, 1H, NH), 8.92 (d, J = 6.3 Hz, 1H, Ar-H), 8.26 (s, 1H, Ar-H), 7.91 (dd, J = 24.9, 11.3 Hz, 3H, Ar-H), 7.58–7.30 (m, 2H, Ar-H), 7.06 (d, J = 8.1 Hz, 1H, Ar-H), 5.28 (s, 1H, NH), 2.45 (s, 3H, –CH3), 1.83–1.36 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 136.85, 134.56, 131.75, 128.50, 123.67, 120.15, 116.05, 111.75, 111.55, 101.72, 56.03, 40.07, 35.58, 28.90, 21.33. HRMS (ESI) calcd for C26H29N4 [M + H]+ 397.2392, found 397.2366.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1267 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 11.18 (s, 1H, NH), 8.35 (dd, J = 39.9, 7.2 Hz, 2H, Ar-H), 8.15 (s, 1H, Ar-H), 7.45 (d, J = 8.8 Hz, 1H, Ar-H), 7.18–7.04 (m, 1H, Ar-H), 6.90 (ddd, J = 35.8, 13.9, 6.9 Hz, 3H, Ar-H), 4.63 (s, 1H, NH), 2.50 (s, 3H, –CH3), 1.87–1.39 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 140.83, 135.41, 123.66, 121.63, 120.19, 119.05, 115.78, 110.68, 109.75, 55.89, 43.32, 35.86, 29.01, 16.64. HRMS (ESI) calcd for C26H29N4 [M + H]+ 397.2392, found 397.2366.
N), 1267 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 11.18 (s, 1H, NH), 8.35 (dd, J = 39.9, 7.2 Hz, 2H, Ar-H), 8.15 (s, 1H, Ar-H), 7.45 (d, J = 8.8 Hz, 1H, Ar-H), 7.18–7.04 (m, 1H, Ar-H), 6.90 (ddd, J = 35.8, 13.9, 6.9 Hz, 3H, Ar-H), 4.63 (s, 1H, NH), 2.50 (s, 3H, –CH3), 1.87–1.39 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 140.83, 135.41, 123.66, 121.63, 120.19, 119.05, 115.78, 110.68, 109.75, 55.89, 43.32, 35.86, 29.01, 16.64. HRMS (ESI) calcd for C26H29N4 [M + H]+ 397.2392, found 397.2366.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1290 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.00 (s, 1H, NH), 8.86 (d, J = 5.8 Hz, 1H, Ar-H), 8.72 (s, 1H, Ar-H), 8.48 (t, J = 20.1 Hz, 1H, Ar-H), 7.82 (s, 1H, Ar-H), 8.00–7.53 (m, 3H, Ar-H), 7.42 (s, 1H, Ar-H), 5.27 (s, 1H, NH), 1.83–1.36 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 185.41, 140.30, 138.81, 125.66, 123.91, 122.61, 119.91, 117.92, 113.97, 113.26, 104.32, 101.56, 56.09, 40.06, 35.61, 28.92. HRMS (ESI) calcd for C26H26N5 [M + H]+ 408.2188, found 408.2158.
N), 1290 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 10.00 (s, 1H, NH), 8.86 (d, J = 5.8 Hz, 1H, Ar-H), 8.72 (s, 1H, Ar-H), 8.48 (t, J = 20.1 Hz, 1H, Ar-H), 7.82 (s, 1H, Ar-H), 8.00–7.53 (m, 3H, Ar-H), 7.42 (s, 1H, Ar-H), 5.27 (s, 1H, NH), 1.83–1.36 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 185.41, 140.30, 138.81, 125.66, 123.91, 122.61, 119.91, 117.92, 113.97, 113.26, 104.32, 101.56, 56.09, 40.06, 35.61, 28.92. HRMS (ESI) calcd for C26H26N5 [M + H]+ 408.2188, found 408.2158.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1242 (C–N). 1H NMR (400 MHz, CDCl3) δ 8.43 (d, J = 7.4 Hz, 2H, Ar-H), 8.17–7.82 (m, 2H, Ar-H), 7.39–7.05 (m, 4H, Ar-H), 6.95 (t, J = 6.8 Hz, 1H, Ar-H), 4.98 (s, 1H, NH), 3.55 (s, 3H, –CH3), 1.81–1.34 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 136.43, 136.36, 132.18, 130.00, 127.10, 124.68, 124.29, 122.19, 121.93, 120.94, 120.73, 115.09, 111.93, 109.47, 100.51, 56.98, 43.55, 35.89, 32.92, 29.45. HRMS (ESI) calcd for C26H29N4 [M + H]+ 397.2392, found 397.2366.
N), 1242 (C–N). 1H NMR (400 MHz, CDCl3) δ 8.43 (d, J = 7.4 Hz, 2H, Ar-H), 8.17–7.82 (m, 2H, Ar-H), 7.39–7.05 (m, 4H, Ar-H), 6.95 (t, J = 6.8 Hz, 1H, Ar-H), 4.98 (s, 1H, NH), 3.55 (s, 3H, –CH3), 1.81–1.34 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 136.43, 136.36, 132.18, 130.00, 127.10, 124.68, 124.29, 122.19, 121.93, 120.94, 120.73, 115.09, 111.93, 109.47, 100.51, 56.98, 43.55, 35.89, 32.92, 29.45. HRMS (ESI) calcd for C26H29N4 [M + H]+ 397.2392, found 397.2366.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1259 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 12.24 (s, 1H, NH), 8.92 (d, J = 6.8 Hz, 1H, Ar-H), 8.48–8.11 (m, 2H, Ar-H), 8.07–7.70 (m, 2H, Ar-H), 7.63–7.38 (m, 2H, Ar-H), 7.24 (dd, J = 8.6, 1.8 Hz, 1H, Ar-H), 5.36 (s, 1H, NH), 1.83–1.35 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 136.95, 134.79, 139.04, 124.46, 120.34, 116.07, 113.67, 111.55, 102.37, 56.16, 40.09, 35.55, 28.90. HRMS (ESI) calcd for C25H26ClN4 [M + H]+ 417.1846, found 417.1814.
N), 1259 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 12.24 (s, 1H, NH), 8.92 (d, J = 6.8 Hz, 1H, Ar-H), 8.48–8.11 (m, 2H, Ar-H), 8.07–7.70 (m, 2H, Ar-H), 7.63–7.38 (m, 2H, Ar-H), 7.24 (dd, J = 8.6, 1.8 Hz, 1H, Ar-H), 5.36 (s, 1H, NH), 1.83–1.35 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 136.95, 134.79, 139.04, 124.46, 120.34, 116.07, 113.67, 111.55, 102.37, 56.16, 40.09, 35.55, 28.90. HRMS (ESI) calcd for C25H26ClN4 [M + H]+ 417.1846, found 417.1814.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1273 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 11.06 (d, J = 1.5 Hz, 1H, NH), 8.36 (dd, J = 28.5, 7.5 Hz, 2H, Ar-H), 8.05 (d, J = 2.4 Hz, 1H, Ar-H), 7.45 (d, J = 8.9 Hz, 1H, Ar-H), 7.17 (s, 1H, Ar-H), 7.15–7.06 (m, 1H, Ar-H), 6.94–6.77 (m, 2H, Ar-H), 4.57 (s, 1H, Ar-H), 2.48 (s, 3H, NH), 1.86–1.39 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 140.96, 136.58, 136.30, 129.99, 124.41, 120.60, 115.89, 111.02, 110.48, 109.49, 55.89, 43.29, 35.86, 29.02, 21.39. HRMS (ESI) calcd for C26H29N4 [M + H]+ 397.2392, found 397.2360.
N), 1273 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 11.06 (d, J = 1.5 Hz, 1H, NH), 8.36 (dd, J = 28.5, 7.5 Hz, 2H, Ar-H), 8.05 (d, J = 2.4 Hz, 1H, Ar-H), 7.45 (d, J = 8.9 Hz, 1H, Ar-H), 7.17 (s, 1H, Ar-H), 7.15–7.06 (m, 1H, Ar-H), 6.94–6.77 (m, 2H, Ar-H), 4.57 (s, 1H, Ar-H), 2.48 (s, 3H, NH), 1.86–1.39 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 140.96, 136.58, 136.30, 129.99, 124.41, 120.60, 115.89, 111.02, 110.48, 109.49, 55.89, 43.29, 35.86, 29.02, 21.39. HRMS (ESI) calcd for C26H29N4 [M + H]+ 397.2392, found 397.2360.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1263 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 11.56 (s, 1H, NH), 8.93 (d, J = 6.6 Hz, 1H, Ar-H), 8.34 (s, 1H, Ar-H), 7.92 (d, J = 7.2 Hz, 3H, Ar-H), 7.53 (dd, J = 32.8, 10.1 Hz, 3H, Ar-H), 6.57 (s, 1H, Ar-H), 5.28 (s, 1H, NH), 1.84–1.38 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 136.63, 136.22, 130.82, 125.94, 120.44, 118.22, 116.21, 111.76, 111.56, 101.77, 56.20, 40.09, 35.56, 28.95. HRMS (ESI) calcd for C25H27N4 [M + H]+ 383.2236, found 383.2205.
N), 1263 (C–N). 1H NMR (400 MHz, DMSO-d6) δ 11.56 (s, 1H, NH), 8.93 (d, J = 6.6 Hz, 1H, Ar-H), 8.34 (s, 1H, Ar-H), 7.92 (d, J = 7.2 Hz, 3H, Ar-H), 7.53 (dd, J = 32.8, 10.1 Hz, 3H, Ar-H), 6.57 (s, 1H, Ar-H), 5.28 (s, 1H, NH), 1.84–1.38 (m, 15H, adamantyl-H). 13C NMR (100 MHz, DMSO-d6) δ 136.63, 136.22, 130.82, 125.94, 120.44, 118.22, 116.21, 111.76, 111.56, 101.77, 56.20, 40.09, 35.56, 28.95. HRMS (ESI) calcd for C25H27N4 [M + H]+ 383.2236, found 383.2205.Anti-HIV-1 activity assays
The inhibitory activity of the compounds against the laboratory-adapted HIV-1IIIB strain was examined using the HIV-1IIIB/TZM-bl indicator cell culture system as previously described.60,61 Briefly, 1.5 × 103 TZM-bl cells were seeded in a volume of 300 μL into each well of a 48-well plate and incubated overnight at 37 °C. The cells were then infected with HIV-1IIIB at a multiplicity of infection (M.O.I.) of 0.1 and incubated in the presence or absence of tested compounds for two days at 37 °C. After that they were lysed in lysis buffer, and the luciferase substrate was added to the cell lysates as recommended by the manufacturer's protocol (Promega). The luciferase activities were quantified using a luciferase reporter assay system (Promega, Madison, WI). The percentage of the compound inhibitory activity was calculated using the following formula: [1 − (E − N)/(P − N)] × 100%, where N represents the negative control, P represents the positive control and E corresponds to the experimental group. The EC50 values (effective concentration for 50% inhibition) were calculated using the computer program Calcusyn. The inhibitory activity of the experimental compounds in primary HIV-1 replication was determined as previously described. Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy donors by standard density gradient centrifugation using a Histopaque-1077 (Sigma). PBMCs were cultured in RPMI 1640 medium containing 15% FBS, 1% penicillin–streptomycin and IL-2 (20 units per mL). Three-day PHA-stimulated (5 μg mL−1) and polybrene-treated (2 μg mL−1) cells were infected with the corresponding primary HIV-1 strain at a M.O.I. of 0.01 in the presence or absence of the tested compounds. The supernatants were collected on day 4 post-infection and tested for p24 antigen expression by ELISA using the RETRO-TEK HIV-1 p24 antigen ELISA kit (USA). The absorbance of the samples at 450 nm (A450) was read using a Tecan GENios microplate reader (Tecan, Switzerland), and the EC50 values were calculated as described above.Acknowledgements
We are grateful to the NSFC (81573279, 81373255, 81560574), the Key Scientific Research Projects of Ministry of Education (313040), Hubei Province's Outstanding Medical Academic Leader Program, and Hunan Province Cooperative Innovation Center for Molecular Target New Drug Study for support of this research.References
- K. Wright, Nature, 1986, 323, 283 CAS.
- J. A. Esté and T. Cihlar, Antiviral Res., 2010, 85, 25–33 CrossRef PubMed.
- D. S. T. David, H. B. Ripin, J. Fortunak, S. M. Basha, N. Bivins, C. N. Boddy, S. Byrn, K. K. Catlin, S. R. Houghton, S. T. Jagadeesh, K. A. Kumar, J. Melton, S. Muneer, L. N. Rao, R. V. Rao, P. C. Ray, N. G. Reddy, R. M. Reddy, K. C. Shekar, T. Silverton, D. T. Smith, R. W. Stringham, G. V. Subbaraju, F. Talley and A. Williams, Org. Process Res. Dev., 2010, 14, 1194–1201 CrossRef.
- W. L. Jorgensen, M. Bollini, V. V. Thakur, R. A. Domaoal, K. A. Spasov and K. S. Anderson, J. Am. Chem. Soc., 2011, 133, 15686–15696 CrossRef CAS PubMed.
- C. Reynolds, C. B. de Koning, S. C. Pelly, W. A. van Otterlo and M. L. Bode, Chem. Soc. Rev., 2012, 41, 4657–4670 RSC.
- G. W. Pratt, A. Fan and C. M. Klapperich, ACS Biomater. Sci. Eng., 2015, 1, 314–319 CrossRef CAS.
- S. Massari, D. Daelemans, M. L. Barreca, A. Knezevich, S. Sabatini, V. Cecchetti, A. Marcello, C. Pannecouque and O. Tabarrini, J. Med. Chem., 2010, 53, 641–648 CrossRef CAS PubMed.
- R. Costi, M. Métifiot, F. Esposito, G. C. Crucitti, L. Pescatori, A. Messore, L. Scipione, S. Tortorella, L. Zinzula, E. Novellino, Y. Pommier, E. Tramontano, C. Marchand and R. D. Santo, J. Med. Chem., 2013, 56, 8588–8598 CrossRef CAS PubMed.
- H. J. Stellbrink, Eur. J. Med.Res., 2007, 12, 483–495 CAS.
- S. Castellino, M. R. Groseclose, J. Sigafoos, D. Wagner, M. D. Serres, J. W. Polli, E. Romach, J. Myer and B. Hamilton, Chem. Res. Toxicol., 2013, 26, 241–251 CrossRef CAS PubMed.
- F. R. Alexandre, A. Amador, S. Bot, C. Caillet, T. Convard, J. Jakubik, C. Musiu, B. Poddesu, L. Vargiu, M. Liuzzi, A. Roland, M. Seifer, D. Standring, R. Storer and C. B. Dousson, J. Med. Chem., 2011, 54, 392–395 CrossRef CAS PubMed.
- P. Vernazza, C. Wang, A. Pozniak, E. Weil, P. Pulik, D. A. Cooper, R. Kaplan, A. Lazzarin, H. Valdez, J. Goodrich, J. Mori, C. Craig and M. Tawadrous, JAIDS, J. Acquired Immune Defic. Syndr., 2013, 62, 171–179 CrossRef CAS PubMed.
- G. Moyle, M. Boffito, A. Stoehr, A. Rieger, Z. Shen, K. Manhard, B. Sheedy, V. Hingorani, A. Raney, M. Nguyen, T. Nguyen, V. Ong, L. T. Yeh and B. Quart, Antimicrob. Agents Chemother., 2010, 54, 3170–3178 CrossRef CAS PubMed.
- J. J. Kennedy-Smith, N. Arora, J. R. Billedeau, J. Fretland, J. Q. Hang, G. M. Heilek, S. F. Harris, D. Hirschfeld, H. Javanbakht, Y. Li, W. Liang, R. Roetz, M. Smith, G. Su, J. M. Suh, A. G. Villasenor, J. Wu, D. Yasuda and Z. K. Sweeney, Med. Chem. Commun., 2010, 1, 79–83 RSC.
- S. M. Gomha, M. G. Badrey, M. M. Abdallac and R. K. Arafa, MedChemComm, 2014, 5, 1685–1692 RSC.
- X. Wang, J. Zhang, Y. Huang, R. Wang, L. Zhang, K. Qiao, L. Li, C. Liu, Y. Ouyang, W. Xu, Z. Zhang, L. Zhang, Y. Shao, S. Jiang, L. Ma and J. Liu, J. Med. Chem., 2012, 55, 2242–2250 CrossRef CAS PubMed.
- K. M. Frey, D. E. Puleo, K. A. Spasov, M. Bollini, W. L. Jorgensen and K. S. Anderson, J. Med. Chem., 2015, 58, 2737–2745 CrossRef CAS PubMed.
- J. Hu, Y. Li, Y. Wu, W. Liu, Y. Wang and Y. Li, Chem. Lett., 2015, 44, 645–647 CrossRef CAS.
- (a) A. Maleki, Helv. Chim. Acta, 2014, 97, 587–593 CrossRef CAS; (b) A. Maleki, Z. Alrezvani and S. Maleki, Catal. Commun., 2015, 69, 29–33 CrossRef CAS; (c) M. J. D. Pires, D. L. Poeira and M. M. B. Marques, Eur. J. Org. Chem., 2015, 7197–7234 CrossRef.
- U. Balijapalli and S. K. Iyer, Dyes Pigm., 2015, 121, 88–98 CrossRef CAS.
- A. Maleki, RSC Adv., 2014, 4, 64169–64173 RSC.
- K. Pericherla, P. Kaswan, K. Pandey and A. Kumar, Synthesis, 2015, 47, 887–912 CrossRef CAS.
- Z. Fei, Y. Zhu, M. Liu, F. Jia and A. Wu, Tetrahedron Lett., 2013, 54, 1222–1226 CrossRef CAS.
- H. Sanaeishoar, R. Nazarpour and F. Mohave, RSC Adv., 2015, 5, 68571–68578 RSC.
- (a) A. Maleki, S. Javanshir and M. Naimabadi, RSC Adv., 2014, 4, 30229–30232 RSC; (b) A. K. Bagdi, M. Rahman, S. Santra, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1741–1747 CrossRef CAS; (c) S. Santra, A. K. Bagdi, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1065–1070 CrossRef CAS; (d) S. Lei, G. Chen, Y. Mai, L. Chen, H. Cai, J. Tan and H. Cao, Adv. Synth. Catal., 2016, 358, 67–73 CrossRef CAS; (e) C. Wang, S. Lei, H. Cao, S. Qiu, J. Liu, H. Deng and C. Yan, J. Org. Chem., 2015, 80, 12725–12732 CrossRef CAS PubMed; (f) H. Cao, S. Lei, N. Li, L. Chen, J. Liu, H. Cai, S. Qiu and J. Tan, Chem. Commun., 2015, 51, 1823–1825 RSC; (g) H. Cao, X. Liu, L. Zhao, J. Cen, J. Lin, Q. Zhu and M. Fu, Org. Lett., 2014, 16, 146–149 CrossRef CAS PubMed; (h) S. Lei, Y. Mai, C. Yan, J. Mao and H. Cao, Org. Lett., 2016, 18, 3582–3585 CrossRef CAS PubMed.
- G. Y. Li, K. H. Jung, H. Lee, M. K. Son, J. Seo, S. W. Hong, Y. Jeong, S. Hong and S. S. Hong, Cancer Lett., 2013, 329, 59–67 CrossRef CAS PubMed.
- R. Ducray, I. Simpson, F. H. Jung, J. W. M. Nissink, P. W. Kenny, M. Fitzek, G. E. Walker, L. T. Ward and K. Hudson, Bioorg. Med. Chem. Lett., 2011, 21, 4698–4701 CrossRef CAS PubMed.
- K. A. Emmitte, B. J. Wilson, E. W. Baum, H. K. Emerson, K. W. Kuntz, K. E. Nailor, J. M. Salovich, S. C. Smith, M. Cheung, R. M. Gerding, K. L. Stevens, D. E. Uehling, R. A. Mook Jr, G. S. Moorthy, S. H. Dickerson, A. M. Hassell, M. A. Leesnitzer, L. M. Shewchuk, A. Groy, J. L. Rowand, K. Anderson, C. L. Atkins, J. Yang, P. Sabbatini and R. Kumar, Bioorg. Med. Chem. Lett., 2009, 19, 1004–1008 CrossRef CAS PubMed.
- C. Jaramillo, J. E. De Diego, C. Hamdouchi, E. Collins, H. Keyser, C. Sánchez-Martínez, M. Del Prado, B. Norman, H. B. Brooks, S. A. Watkins, C. D. Spencer, J. A. Dempsey, B. D. Anderson, R. M. Campbell, T. Legget, B. Patel, R. M. Schultz, J. Espinosa, M. Vieth, F. Zhang and D. E. Timm, Bioorg. Med. Chem. Lett., 2004, 14, 6095–6099 CrossRef CAS PubMed.
- C. Hamdouchi, J. Ezquerra, J. A. Vega, J. J. Vaquero, J. Alvarez-Builla and B. A. Heinz, Bioorg. Med. Chem. Lett., 1999, 9, 1391–1394 CrossRef CAS PubMed.
- M. Lhassani, O. Chavignon, J. M. Chezal, J. C. Teulade, J. P. Chapat, R. Snoeck, G. Andrei, J. Balzarini, E. D. Clercq and A. Gueiffier, Eur. J. Med. Chem., 1999, 34, 271–274 CrossRef CAS.
- S. Mavel, J. L. Renou, C. Galtier, H. Allouchi, R. Snoeck, G. Andrei, E. D. Clercq, J. Balzarini and A. Gueiffier, Bioorg. Med. Chem., 2002, 10, 941–946 CrossRef CAS PubMed.
- J. B. Veron, H. Allouchi, C. Enguehard-Gueiffier, R. Snoeck, G. Andrei, E. D. Clercq and A. Gueiffier, Bioorg. Med. Chem., 2008, 16, 9536–9545 CrossRef CAS PubMed.
- N. M. Shukla, D. B. Salunke, E. Yoo, C. A. Mutz, R. Balakrishna and S. A. David, Bioorg. Med. Chem., 2012, 20, 5850–5863 CrossRef CAS PubMed.
- T. H. Al-Tel, R. A. Al-Quwasmeh and R. Zaarour, Eur. J. Med. Chem., 2011, 46, 1874–1881 CrossRef CAS PubMed.
- J. T. Starr, R. J. Sciotti, D. L. Hanna, M. D. Huband, L. M. Mullins, H. Cai, J. W. Gage, M. Lockard, M. R. Rauckhorst, R. M. Owen, M. S. Lall, M. Tomilo, H. Chen, S. P. McCurdy and M. R. Barbachyn, Bioorg. Med. Chem. Lett., 2009, 19, 5302–5306 CrossRef CAS PubMed.
- A. Scribner, R. Dennis, S. Lee, G. Ouvry, D. Perrey, M. Fisher, M. Wyvratt, P. Leavitt, P. Liberator, A. Gurnett, C. Brown, J. Mathew, D. Thompson, D. Schmatz and T. Biftu, Eur. J. Med. Chem., 2008, 43, 1123–1151 CrossRef CAS PubMed.
- B. F. McGuiness, A. W. Cole, G. Dong, M. R. Brescia, Y. Shao, I. Henderson, L. L. Rokosz, T. M. Stauffer, N. Mannava, E. F. Kimble, C. Hicks, N. White, P. G. Wines and E. Quadros, Bioorg. Med. Chem. Lett., 2010, 20, 6845–6849 CrossRef PubMed.
- W. M. El-Sayed, W. A. Hussin, Y. S. Al-Faiyz and M. A. Ismail, Eur. J. Pharmacol., 2013, 715, 212–218 CrossRef CAS PubMed.
- M. Frohn, V. Viswanadhan, A. J. Pickrell, J. E. Golden, K. M. Muller, R. W. Bürli, G. Biddlecome, S. C. Yoder, N. Rogers, J. H. Dao, R. Hungate and J. R. Allen, Bioorg. Med. Chem. Lett., 2008, 18, 5023–5026 CrossRef CAS PubMed.
- S. Ulloora, R. Shabaraya, S. Aamir and A. V. Adhikari, Bioorg. Med. Chem. Lett., 2013, 23, 1502–1506 CrossRef CAS PubMed.
- N. Bailey, M. J. Bamford, D. Brissy, J. Brookfield, E. Demont, R. Elliott, N. Garton, I. Farre-Gutierrez, T. Hayhow, G. Hutley, A. Naylor, T. A. Panchal, H. X. Seow, D. Spalding and A. K. Takle, Bioorg. Med. Chem. Lett., 2009, 19, 3602–3606 CrossRef CAS PubMed.
- P. J. Zimmermann, W. Buhr, C. Brehm, A. M. Palmer, M. P. Feth, J. Senn-Bilfinger and W. A. Simon, Bioorg. Med. Chem. Lett., 2007, 17, 5374–5378 CrossRef CAS PubMed.
- U. Grädler, T. Fuchß, W. R. Ulrich, R. Boer, A. Strub, C. Hesslinger, C. Anézo, K. Diederichs and A. Zaliani, Bioorg. Med. Chem. Lett., 2011, 21, 4228–4232 CrossRef PubMed.
- N. Dahan-Farkas, C. Langley, A. L. Rousseau, D. B. Yadav, H. Davids and C. B. de Koning, Eur. J. Med. Chem., 2011, 46, 4573–4583 CrossRef CAS PubMed.
- M. L. Bode, D. Gravestock, S. S. Moleele, C. W. van der Westhuyzen, S. C. Pelly, P. A. Steenkamp, H. C. Hoppe, T. Khan and L. A. Nkabinde, Bioorg. Med. Chem., 2011, 19, 4227–4237 CrossRef CAS PubMed.
- L. Wanka, K. Iqba and P. R. Schreiner, Chem. Rev., 2013, 113, 3516–3604 CrossRef CAS PubMed.
- N. Kolocouris, A. Kolocouris, G. B. Foscolos, G. Fytas, J. Neyts, E. Padalko, J. Balzarini, R. Snoeck, G. Andrei and E. De Clercq, J. Med. Chem., 1996, 39, 3307–3318 CrossRef CAS PubMed.
- G. Fytas, G. Stamatiou, G. B. Foscolos, A. Kolocouris, N. Kolocouris, M. Witvrouw, C. Pannecouque and E. De Clercq, Bioorg. Med. Chem. Lett., 1997, 7, 1887–1890 CrossRef CAS.
- G. Stamatiou, G. B. Foscolos, G. Fytas, A. Kolocouris, N. Kolocouris, C. Pannecouque, M. Witvrouw, E. Padalko, J. Neyts and E. De Clercq, Bioorg. Med. Chem., 2003, 11, 5485–5492 CrossRef CAS PubMed.
- N. Kolocouris, A. Kolocouris, G. B. Foscolos, G. Fytas, J. Neyts, E. Padalko, J. Balzarini, R. Snoeck, G. Andrei and E. D. Clercq, J. Med. Chem., 1996, 39, 3307–3318 CrossRef CAS PubMed.
- M. E. Burstein, A. V. Serbin, T. V. Khakhulina, I. V. Alymova, L. L. Stotskaya, O. P. Bogdan, E. E. Manukchina, V. V. Jdanov, N. K. Sharova and A. G. Bukrinskaya, Antiviral Res., 1999, 41, 135–144 CrossRef CAS PubMed.
- J. Balzarini, B. Orzeszko-Krzesinska, J. K. Maurin and A. Orzeszko, Eur. J. Med. Chem., 2009, 44, 303–311 CrossRef CAS PubMed.
- X. Han, N. Sun, H. Wu, D. Guo, P. Tien, C. Dong, S. Wu and H. B. Zhou, J. Med. Chem., 2016, 59, 2139–2150 CrossRef CAS PubMed.
- J. Pan, X. Han, N. Sun, H. Wu, D. Lin, P. Tien, H. B. Zhou and S. Wu, RSC Adv., 2015, 5, 55100–55108 RSC.
- X. Han, C. Dong and H. B. Zhou, Adv. Synth. Catal., 2014, 356, 1275–1280 CrossRef CAS.
- X. Han, H. Wu, W. Wang, C. Dong, P. Tien, S. Wu and H. B. Zhou, Org. Biomol. Chem., 2014, 12, 8308–8317 CAS.
- X. Han, W. Ouyang, B. Liu, W. Wang, P. Tien, S. Wu and H. B. Zhou, Org. Biomol. Chem., 2013, 11, 8463–8475 CAS.
- X. Han, H. Wu, C. Dong, P. Tien, W. Xie, S. Wu and H. B. Zhou, RSC Adv., 2015, 5, 10005–10013 RSC.
- A. A. El-Emam, O. A. Al-Deeb, M. A. Al-Omar and J. Lehmann, Bioorg. Med. Chem., 2004, 12, 5107–5113 CrossRef CAS PubMed.
- J. Balzarini, B. Orzeszko, J. K. Mauri and A. Orzeszko, Eur. J. Med. Chem., 2007, 42, 993–1003 CrossRef CAS PubMed.
- D. B. Salunke, E. Yoo, N. M. Shukla, R. Balakrishna, S. S. Malladi, K. J. Serafin, V. W. Day, X. Wang and S. A. David, J. Med. Chem., 2012, 55, 8137–8151 CrossRef CAS PubMed.
- G. Zhou, D. Wu, B. Snyder, R. G. Ptak, H. Kaur and M. Gochin, J. Med. Chem., 2011, 54, 7220–7231 CrossRef CAS PubMed.
- J. D. Bauman, D. Patel, C. Dharia, M. W. Fromer, S. Ahmed, Y. Frenkel, R. S. Vijayan, J. T. Eck, W. C. Ho, K. Das, A. J. Shatkin and E. Arnold, J. Med. Chem., 2013, 56, 2738–2746 CrossRef CAS PubMed.
- M. L. Bode, A. L. Rousseau, D. Gravestock, S. S. Moleele and C. W. van der Westhuyzen, European Patent WO 2010/032195, 2010.
- T. An, W. Ouyang, W. Pan, D. Guo, J. Li, L. Li, G. Chen, J. Yang, S. Wu and P. Tien, Antiviral Res., 2012, 94, 276–287 CrossRef CAS PubMed.
- M. L. Bode, D. Gravestock, S. S. Moleele, C. W. van der Westhuyzen, S. Pelly, P. A. Steenkamp, H. C. Hoppe, T. Khan and L. A. Nkabinde, Bioorg. Med. Chem., 2011, 19, 4227–4237 CrossRef CAS PubMed.
- O. Trott and A. J. Olson, J. Comput. Chem., 2010, 31, 455–461 CAS.
- T. An, W. Ouyang, W. Pan, D. Guo, J. Li, L. Li, G. Chen, J. Yang, S. Wu and P. Tien, Antiviral Res., 2012, 94, 276–287 CrossRef CAS PubMed.
- J. J. Martinez-Irujo, M. L. Villahermosa, J. Mercapide, J. F. Cabodevill and E. Santiago, Biochem. J., 1998, 329, 689–698 CrossRef CAS PubMed.
Footnote |
| † These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |