Synthesis, characterization, and air stability study of pyrimido[4,5-g]quinazoline-4,9-dione-based polymers for organic thin film transistors†
Jesse Quinn,
Yinghui He,
Daid A. Khan,
Jonathan Rasmussen,
Haritosh Patel,
Fezza Haider,
Wasim Kapadia and
Yuning Li*
Department of Chemical Engineering, Waterloo Institute of Nanotechnology, (WIN), University of Waterloo, 200 University Ave West, Waterloo, Ontario N2L 3G1, Canada. E-mail: yuning.li@uwaterloo.ca; Fax: +1-519-888-4347; Tel: +1-519-888-4567 ext. 31105
First published on 12th August 2016
Abstract
We report two novel π-conjugated polymers, PPQ2T-TVT-24 and PPQ2T-TT-24, containing the pyrimido[4,5-g]quinazoline-4,9-dione moiety and thieno[3,2-b]thiophene (TT) and (E)-1,2-bis(thiophen-2-yl)ethene (TVT), respectively, via the Stille cross-coupling reaction. Both polymers displayed good thermal stability and promising field effect transistor performance with hole mobilities of up to 3.08 × 10−3 cm2 V−1 s−1 for PPQ2T-TT-24 and 5.34 × 10−3 cm2 V−1 s−1 for PPQ2T-TVT-24. The ambient air stability of the organic thin film transistors based on these two polymers along with another previously reported PQ polymer containing bithiophene (BT) units, PPQ2T-BT-24, was studied. It was found that the moisture (H2O) in the ambient air is a detrimental component responsible for the degradation of the device performance, while oxygen, in contrast, could enhance the carrier mobility. Our study showed that the electron donor comonomer unit significantly influenced the stability of these polymers towards moisture in the stability order of TT > BT ≫ TVT. It was shown that the interaction between H2O and PPQ2T-TT-24 is physisorption and the device performance could be fully recovered, while the interaction of H2O with two other polymers involved chemical reactions, leading to permanent damages to the polymers and only partially recovered device performance upon removal of moisture.
Introduction
Semiconducting polymers have been studied for over three decades in devices such as light emitting diodes, photovoltaic cells, and thin-film transistors. The strong interest to integrate polymer semiconductors in these devices originates from their attractive processing ability, diverse substrate compatibilities, versatile structural tunability, and low device fabrication costs.1–6 Great effort has been made to develop new polymer semiconductors for organic thin film transistors (OTFTs) in order to improve their overall performance, especially charge carrier mobility and air stability,7–12 two performance parameters that are key for many applications. Among the high performance polymers reported for OTFTs, the ones comprised of amide/imide containing building blocks13 are of particular interest and most widely studied recently due to several reasons: (a) the strong electron withdrawing amide/imide group can lower the lowest unoccupied molecular orbital (LUMO) energy level of the polymer to stabilize electron transport; (b) the amide/imide building blocks can also lower the highest occupied molecular orbital (HOMO) level to stabilize the hole transport; (c) the N-alkylation of amide/imide groups provides a tool to render the polymer solution processable; (d) the N-alkyl chains are usually distant from aromatic cores, minimizing the steric hindrance to maintain coplanarity of the polymer backbone for efficient intramolecular charge transport.Recently, we reported a new amide containing building block, pyrimido[4,5-g]quinazoline-4,9-dione (PQ),14,15 for polymer semiconductors. Our research into PQ polymers is driven by the known bioactivities of PQ derivatives that have been studied as anti-microbial, anti-tumour, anti-inflammatory, anti-convulsant, anti-hypertensive, and anti-malarial agents.16–23 Our previous results showed that the copolymers of PQ and bithiophene (PPQ2T-BT-24 shown in Fig. 1 and PPQ2T-BT-40) are highly sensitive to acids, which can potentially be used as active channel layers in OTFT based bio- and chemo-sensors.14 These two polymers demonstrated modest charge carrier performance (∼10−3 cm2 V−1 s−1) in OTFTs. It is known that the comonomer to the amide/imide-containing monomer is critical for charge transport performance. For example, for DPP-based polymers, the charge carrier mobility values vary from ∼10−3 cm2 V−1 s−1 to 17.8 cm2 V−1 s−1 depending on the chosen co-monomer unit, which can regulate the frontier molecular orbital energy levels, molecular packing, and thin-film morphology.24–28 Thieno[3,2-b]thiophene (TT) and (E)-1,2-bis(thiophen-2-yl)ethene (TVT) have been used as donor building blocks to couple with the electron-withdrawing amide/imide building blocks forming donor–acceptor (D–A) polymers that exhibit very high charge carrier mobility values,29–31 sometimes greater than those of BT-containing polymers. To further explore the potential of PQ as a building block in polymer semiconductors, in this work, we selected these two comonomer units to make two novel PQ-based π-conjugated polymers, PPQ2T-TT-24 and PPQ2T-TVT-24, respectively (Scheme 1). When used as active channel layers in OTFTs, PPQ2T-TT-24 and PPQ2T-TVT-24 demonstrated charge carrier mobilities of ∼10−3 cm2 V−1 s−1 that are similar to that of PPQ2T-BT-24 under nitrogen atmosphere. When the devices were operated in the ambient air (with 52% relative humidity, RH), PPQ2T-TT-24 exhibited better stability than PPQ2T-TVT-24 and PPQ2T-BT-24. To decouple the influences of air and moisture (H2O) in the ambient air on device performance, three PQ polymers were tested under dry air and moist nitrogen atmospheres. It was shown that moisture in the air has a detrimental effect on the hole mobility with differing mechanisms for three polymers. Surprisingly, the dry air (or oxygen) improved the hole mobility of these polymers.
 | ||
| Fig. 1 Structure of PPQ2T-BT-24.14 | ||
Results and discussion
Fig. 2 shows the optimized geometry at ground state with LUMO and HOMO wavefunctions for PQ2T-TT-Me and PQ2T-TVT-Me dimers, respectively, obtained by density functional theory (DFT) at the B3LYP 6-31G(d) level of theory. As observed in our previous findings,14 the dihedral angle between the PQ moiety and the flanking thiophene unit is ∼26°, which is considered a limiting factor for achieving a high coplanarity (ESI†). The calculated frontier energy levels, LUMO/HOMO, for PQ2T-TT-Me and PQ2T-TVT-Me dimers are −2.57 eV/−5.26 eV and −2.62 eV/−5.04 eV, respectively. The LUMO wavefunction localizes at TT (or TVT) and the flanking thiophene units and thus give relatively high LUMO energy levels, whereas the HOMO wavefunction is delocalized along the dimer backbones across the PQ and TT (or TVT) units. In comparison with the LUMO/HOMO of PQ2T-BT-Me (−2.57 eV/−5.17 eV),14 it appears that the HOMO levels changed notably with differing donor comonomer units (HOMO: TT < BT < TVT), while their LUMO levels remain similar.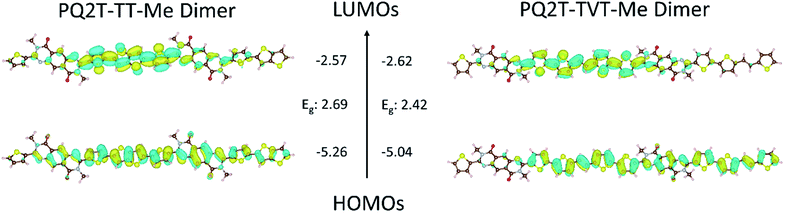 | ||
| Fig. 2 Orbital density distribution (wavefunctions) for the frontier molecular orbitals of PQ2T-TT-Me and PQ2T-TVT-Me dimers. DFT calculations were performed using the Gaussian 09 suite of programs and the B3LYP/6-31G(d) level of theory.32 Theoretical HOMO/LUMO energy levels and band-gaps (vs. vacuum scale) are also shown. | ||
Polymers PPQ2T-TT-24 and PPQ2T-TVT-24 were synthesized via Stille coupling polymerization of 2,7-bis(5-bromothiophen-2-yl)-3,8-bis(2-decyltetradecyl)-3,8-dihydropyrimido[4,5-g]quinazoline-4,9-dione15 with 2,5-bis(trimethylstannyl)thieno[3,2-b]thiophene and 1,2-bis(5-(trimethylstannyl)thiophen-2-yl)ethene, respectively (Scheme 1). The polymers were purified using Soxhlet extraction sequentially with acetone, hexanes, and chloroform. The chloroform fraction was then concentrated by removing some solvent and precipitated from methanol. After drying, PPQ2T-TT-24 and PPQ2T-TVT-24 were obtained in 82% and 87% yields, respectively. The number average molecular weight (Mn) and polydispersity index (PDI) were measured to be 18.3 kg mol−1 and 2.23 for PPQ2T-TT-24 and 19.4 kg mol−1 and 2.52 for PPQ2T-TVT-24, respectively (ESI†). Data were obtained using high-temperature gel-permeation chromatography (HT-GPC) at 140 °C with 1,2,4-trichlorobenzene as an eluent and polystyrene as the standards. The thermal properties of the polymers were characterized by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) (ESI†). PPQ2T-TT-24 and PPQ2T-TVT-24 showed 5% weight loss temperatures (T−5%) of 366 °C and 376 °C, respectively, on their TGA thermograms. Both polymers showed visible glass transition temperatures at ∼60–70 °C on their DSC thermograms.
To elucidate the polymer chain ordering, XRD measurements were performed with polymer thin films spin coated on a DDTS-modified SiO2/Si wafer and annealed at different temperatures (Fig. 3). At an annealing temperature of 80 °C, a primary (100) diffraction peak at 2θ = 4.03°, which corresponds to a d-spacing distance of 2.19 nm, and a low intensity secondary (200) diffraction peak (2θ = 8.22°) were observed for PPQ2T-TT-24. A slight increase in the intensity for the (100) and (200) peaks occurred at an annealing temperature of 100 °C, whereas further increasing the annealing temperature results in a gradual increase in the intensity of the (100) and (200) peaks. At an annealing temperature of 250 °C and above, a decrease in the peak intensity was observed. Since the TGA data show that these polymers should be stable at 250 and 300 °C and the UV-vis spectra of the films showed no significant changes in the 250 °C- and 300 °C-annealed films compared with films annealed at lower temperatures (see Fig. S9 in the ESI†), this reduction of the peak intensity might be caused by the creation of pin-holes at these high temperatures (see the AFM images in Fig. 5), which reduced the coverage of the substrate by the polymer film. For the PPQ2T-TVT-24 thin film annealed at 80 °C, a weak (100) peak at 2θ = 4.39° (d-spacing = 2.01 nm) appeared. With increasing annealing temperature, the intensity of the (100) peak grew, along with the appearance of a secondary (200) diffraction peak (2θ = 8.58°). For the 250 °C- and 300 °C-annealed films, a reduction in the peak intensity was also observed due probably to the formation of pin-holes discussed as above for PPQ2T-TT-24.
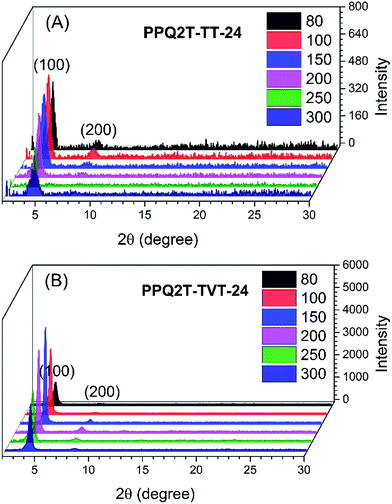 | ||
| Fig. 3 XRD patterns of PPQ2T-TT-24 (A) and PPQ2T-TVT-24 (B) thin films after annealing in nitrogen at different temperatures, respectively. | ||
The UV-vis absorption spectra of PPQ2T-TT-24 and PPQ2T-TVT-24 in chloroform and in thin films are shown in Fig. 4. PPQ2T-TT-24 showed a wavelength of maximum absorption (λmax) at 522 nm (along with a shoulder at ∼548 nm) for solution and 521 nm (along with a shoulder at ∼556 nm) for the thin film. PPQ2T-TVT-24 showed a similar absorption pattern with the λmax at 537 nm (along with a shoulder at ∼571 nm) in solution and 536 nm (along with a shoulder at ∼575 nm) for the thin-film. The more resolvable vibronic splitting or the more pronounced absorption shoulders at the long wavelength side of the spectra of the thin film compared to the solution spectra are characteristic of more ordered polymer chains in the solid state33–35 and were observed for our previously reported PQ-based polymer PPQ2T-BT-24.14 The optical band gaps (Eoptg) were calculated from the UV-vis absorption spectra edge and found to equal 2.0 eV and 1.9 eV for PPQ2T-TT-24 and PPQ2T-TVT-24, respectively, which are similar to the Eoptg of PPQ2T-BT-24 (2.0 eV). The experimental Eoptg is in good agreement with the expected trend observed in the computational study. UV-vis spectra of the films of these two polymers annealed at temperatures up to 300 °C showed no noticeable changes, indicating their excellent thermal stability (Fig. S9†).
 | ||
| Fig. 4 UV-vis absorption spectra of PPQ2T-TT-24 and PPQ2T-TVT-24 as thin films and in chloroform solution (inset). | ||
The morphological changes of the polymer thin films were examined by atomic force microscopy (AFM) (Fig. 5). The PPQ2T-TT-24 film annealed at temperatures lower than 200 °C showed large particles (irregular shaped bright spots) of a few hundred nanometers in size. The formation of large particles might be due to the poor solubility of this polymer that caused precipitation of polymer aggregates as solvent evaporated during spin coating. As the annealing temperature was increased to 250 °C, these particles disappeared accompanied with the formation of pin-holes (dark spots). The number of pin-holes increased as the annealing temperature was increased to 300 °C. The formation of pin-holes is likely due to the weakened interfacial interaction between the polymer film and the substrate at high temperatures. The 80 °C-annealed PPQ2T-TVT-24 film showed some round-shaped particles (bright spots) and well-defined grains. Similar to PPQ2T-TT-24, the number of (bright) particles decreases with increasing annealing temperature. The particles almost disappeared at the annealing temperature of 200 °C, 50 °C lower compared with PPQ2T-TT-24. However, pin-holes also starts to appear at 200 °C, indicating that the interfacial interaction between the PPQ2T-TVT-24 film and the substrate is weaker than that between PPQ2T-TVT-24 and the substrate.
 | ||
| Fig. 5 AFM images (4 μm × 4 μm) of PPQ2T-TT-24 and PPQ2T-TVT-24 thin films after annealing at desired temperatures. | ||
Cyclic voltammetry (CV) measurements were conducted on the polymer thin-films (ESI†) using ferrocene as a reference with a reported HOMO energy level of −4.8 eV against vacuum.36 The HOMO energy levels are thus calculated to be −5.42 eV and −5.18 eV for PPQ2T-TT-24 and PPQ2T-TVT-24, falling below and above that of PPQ2T-BT-24 (−5.30 eV), respectively, which are also in good agreement with the trend observed in the computational study. It should be noted that most of the p-type polymer semiconductors with high hole mobility have a relatively high HOMO level between −5.1 eV and −5.3 eV.30,37,38 Therefore, the HOMO level of PPQ2T-TVT-24 should be more favoured for hole injection and transport. No peaks were observed during the reduction cycles, so the LUMO levels were calculated from the HOMO levels and Eoptg to be −3.41 eV and −3.27 eV for PPQ2T-TT-24 and PPQ2T-TVT-24 respectively.
A bottom-gate bottom-contact (BGBC) transistor architecture was used to evaluate the charge carrier transport characteristics for each polymer. n++-Doped conductive silicon wafer with a SiO2 dielectric layer (∼300 nm thick) was used as the substrate. Both polymers only showed hole transport performance due to their relatively high LUMO levels. A LUMO level of ∼−3.7 eV to ∼−4.0 eV or deeper is required for stable n-type performance.39–41 PPQ2T-TT-24 devices showed the best performance with the 300 °C-annealed films reaching a maximum hole mobility of 3.08 × 10−3 cm2 V−1 s−1 (Fig. 6A) and an average mobility of 2.33 × 10−3 cm2 V−1 s−1 (Table S1†). PPQ2T-TVT-24 devices performed the best for the 150 °C-annealed films reaching a maximum hole mobility of 5.34 × 10−3 cm2 V−1 s−1 (Fig. 6B) and an average mobility of 4.81 × 10−3 cm2 V−1 s−1 (Table S1 in the ESI†). At an annealing temperature greater than 200 °C, the mobility values dropped. This mobility reduction trend coincidently agrees with the formation of pin-holes as observed in the AFM images (Fig. 5). These pin-holes would interrupt the charge transport pathways. As afore-mentioned, the formation of pin-holes is due to the weakened polymer–substrate interface, which would also negatively influence the charge carrier mobility due to the poorer polymer–dielectric contact.42–44
 | ||
| Fig. 6 Output (left) and transfer (right) curves for OTFT devices using PPQ2T-TT-24 (A) and PPQ2T-TVT-24 (B) under nitrogen annealed at 300 °C and annealed at 150 °C, respectively. | ||
The devices using polymer films annealed at 200 °C were subject to testing in the ambient air (with 52% RH) after testing in dry nitrogen in a glove box. The PPQ2T-TT-24 based devices measured in the ambient air showed a decrease of 18% in the average mobility with respect to that measured in dry nitrogen (Fig. 7A). On the other hand, the mobility of the PPQ2T-TVT-24 based devices dropped almost completely (∼94%) upon exposing to the ambient air. We also tested the air stability of devices with the previously reported polymer PPQ2T-BT-24, which showed a decrease of ∼49% in mobility compared with the value measured in dry nitrogen.
 | ||
| Fig. 7 Hole mobility comparison for OTFT devices using different polymers in nitrogen and subsequently in air (∼52% relative humidity) consecutively (A) and under specific environments (B). See ESI† for voltage threshold comparisons. | ||
These devices were then moved back to the nitrogen-filled glove box, baked at 100 °C for 1 h to remove adsorbed moisture and oxygen, and tested again. The average mobility of the PPQ2T-BT-24 based devices recovered by 70% of the value initially measured in the glove box in dry nitrogen (Fig. 7A). The PPQ2T-TVT-24 based devices showed negligible field effect performance, indicating that once these devices were measured in the ambient air, they underwent permanent damages. Interestingly, the PPQ2T-TT-24 based devices exhibited a 17% increase in the average mobility compared to that obtained initially in dry nitrogen. It has been reported that numerous factors might influence the ambient stability of OTFT materials/devices,45–47 which is still a subject of intense research.48–51 As reported by Lu et al.,52 the ambient stability of polymer semiconductors in regard to oxidative doping is governed by the ionization potential or the HOMO energy level of the polymers. This is evident by the trend observed in this study (Fig. 7A) where PPQ2T-TT-24 with the deepest HOMO level of −5.42 eV shows the greatest stability, while PPQ2T-TVT-24 with the highest HOMO level of −5.18 eV shows the poorest stability in the ambient air. Since air is composed of nitrogen, oxygen, water vapour, and small amounts of other gases, it is reasonable to assume that oxygen and moisture (H2O) are the two major components that may degrade the device performance of these polymers. It has been reported that dry oxygen had little effect on the field effect characteristics54 or some enhancement effects on the saturation current and mobility55 of pentacene based OTFTs. It was also found that the absorbed H2O at the grain boundaries of the pentacene film could either decrease the mobility by trapping charge carriers and altering the film morphology53,54 or increase the mobility by forming water clusters.54,55 To gain a better understanding of the influences of air (free of moisture) and moisture (free of oxygen) separately on the performance of our polymers, devices were characterized sequentially under dry nitrogen (in a glove box), dry air (in a container filled with dry air), dry nitrogen (in the same glove box), moist nitrogen (in a container filled with nitrogen saturated with water vapor), and finally in dry nitrogen (in a glove box) (Fig. 7B). For all three polymers, their devices showed increased hole mobilities in dry air with the largest increase by ∼33% observed for PPQ2T-TT-24, indicating dry oxygen is not harmful but beneficial for the hole transport, which agrees with the observation in a report on pentacene OTFTs.55 This enhancement in hole mobility, which was also observed for another p-type polymer reported by our group,51 can be interpreted by the increased work function by oxygen.24 For the pentacene devices, the increases in the saturation current and mobility were attributed to the formation of a charge transfer complex (or p-doping) between pentacene and O2.55 However, it is unlikely the case for our polymers, because PPQ2T-TT-24, which has the deepest HOMO level, or is the most difficult to be p-doped by O2, showed the largest enhancement in mobility among three polymers (Fig. 7B).
Once the devices were returned to the glove box for re-testing under dry nitrogen, the mobilities dropped to similar levels obtained in the first measurement in dry nitrogen. This further demonstrated that dry oxygen did not damage the polymers. Once the devices were taken to the moist nitrogen atmosphere and tested, the mobilities of PPQ2T-TT-24 and PPQ2T-BT-24 dropped by 15% and 19%, respectively. The PPQ2T-TVT-24 devices showed the largest drop in mobility by 43%. To examine if the effect of moisture on the device performance is reversible, the devices were measured in the N2-filled glove box again (for the 3rd time). It was shown that the average mobility of the PPQ2T-TT-24 based devices fully recovered to the value obtained in the first and second measurements in dry N2 (Fig. 7B). On the other hand, the PPQ2T-BT-24 and PPQ2T-TVT-24 based devices partially recovered by ∼90% and ∼50% of the mobilities obtained before exposing to the moist N2, respectively, indicating that part of the performance degradation in moist nitrogen is permanent for these two polymers. These results manifest that water, not oxygen, in the ambient air has a detrimental effect on the hole transport performance of these polymers. Our study also showed that different donor comonomers have dramatic influences on the ambient air stability or more precisely the stability towards H2O. The TT-containing polymer, PPQ2T-TT-24, showed the best stability in the ambient air and the device performance can be recovered once the devices were returned to a dry nitrogen atmosphere, which indicates that the drop in mobility for this polymer is mainly caused by physisorption of water molecules that might function as hole traps. On the other hand, PPQ2T-BT-24 and PPQ2T-TVT-24, in particular, undergo chemical reactions with water, causing permanent damages to the polymers and the device performance cannot be fully recovered. Since these polymers are stable when stored in the ambient air, the chemical reactions of these polymers with water presumably occur under a positively charged state during the device operation in the hole accumulation mode.
Conclusions
We report two novel π-conjugated polymers, PPQ2T-TT-24 and PPQ2T-TVT-24, containing pyrimido[4,5-g]quinazoline-4,9-dione (PQ) and the electron donor comonomer units, thieno[3,2-b]thiophene (TT) and (E)-1,2-bis(thiophen-2-yl)ethene (TVT), respectively. The UV-vis absorption characteristics, frontier energy levels, crystallinity, as well as thin film morphology were found to vary notably with different donor units. Both polymers showed typical hole transport performance as channel semiconductors in OTFTs with mobilities as high as 3.08 × 10−3 cm2 V−1 s−1 for PPQ2T-TT-24 and 5.34 × 10−3 cm2 V−1 s−1 for PPQ2T-TVT-24. Device stability of these two polymers together with another previously reported PQ polymer, PPQ2T-TT-24, was systematically investigated in different atmospheres: dry nitrogen, moist nitrogen, dry air, and ambient air (∼52% RH). It was found that oxygen could enhance the hole mobility of these polymers, which was interpreted by the increased work function of the gold contact electrodes that facilitated hole injection. On other hand, moisture (H2O) notably decreased the mobility of all three polymers. Interestingly, the device performance of PPQ2T-TT-24 could be fully recovered, indicating the interaction between H2O and this polymer is physisorption and the decrease in mobility is due to trapping of charge carriers. For PPQ2T-BT-24 and PPQ2T-TVT-24, the mobilities of their OTFT devices that were tested in the presence of moisture could only be partially recovered when measured in dry N2 again, which indicates that the interaction of H2O with these two polymers involved chemical reactions leading to permanent damages of the polymer structures during device operation. Our results revealed that the electron donor comonomer in a PQ polymer and possibly in other D–A polymers has a significant effect on the ambient air stability or more precisely the stability towards the moisture, with the stability order of TT > BT ≫ TVT. Our study provided useful guidance on the design of ambient air (or moisture) stable polymer semiconductors for printed electronics by choosing appropriate donor monomer building blocks.Experimental
Materials and instrumentation
Chemical reagents were purchased from Sigma Aldrich and used without further purification. 2,7-Bis(5-bromothiophen-2-yl)-3,8-bis(2-decyltetradecyl)-3,8-dihydropyrimido[4,5-g]quinazoline-4,9-dione (PQ2T-24-Br) was synthesized according to our previously reported method.15 NMR data were recorded on a Bruker DPX 300 MHz spectrometer with chemical shifts relative to the residual CHCl3 in the deuterated solvent (7.26 ppm for CHCl3).56 GPC measurements were performed on a Malvern SEC system using 1,2,4-trichlorobenzene as eluent and polystyrene as standards at 140 °C. TGA measurements were carried out on a TA Instruments Q500 at a temperature ramping rate of 10 °C min−1 under nitrogen atmosphere. DSC measurements were carried out on a TA Instruments Q2000 at a temperature ramping rate of 20 °C min−1 under nitrogen atmosphere. The UV-vis absorption spectra of polymers were recorded on a Thermo Scientific GENESYS™ 10S VIS spectrophotometer. Cyclic voltammetry (CV) data were obtained on a CHI600E electrochemical analyzer using an Ag/AgCl reference electrode and two platinum disk electrodes as the working and counter electrodes in a 0.1 M tetrabutylammonium hexafluorophosphate (Bu4NPF6) anhydrous acetonitrile solution at a scan rate of 50 mV s−1. Ferrocene acted as a reference with a reported HOMO energy level of −4.8 eV.36 Thin-film XRD diagrams of polymer thin-films (∼40 nm) spin-coated on dodecyltrichlorosilane (DDTS) modified SiO2/Si substrates and annealed at chosen temperatures for 20 min under nitrogen atmosphere were obtained using a Bruker D8 Advance powder diffractometer with Cu Kα1 radiation (λ = 0.15406 nm). Atomic force microscopy (AFM) images were taken on polymer thin films spin-coated on the DDTS modified SiO2/Si substrates with a Dimension 3100 scanning probe microscope. Density functional theory (DFT) calculations were performed through Gaussian 09 Revision D.01 (ref. 32) using the functional B3LYP and the basis set 6-31G(d) under tight convergence to investigate the geometry, molecular energy levels, and electron distributions for the model compounds.Synthesis of PPQ2T-TT-24
A 25 mL dry Schlenk flask was charged with PQ2T-24-Br (0.10 g, 0.09 mmol), 2,5-bis(trimethylstannyl)thieno[3,2-b]thiophene (0.040 g, 0.09 mmol), and tri(o-tolyl)phosphine (P(o-tolyl)3) (2 mg, 0.01 mmol). After degassing and refilling argon three times, anhydrous chlorobenzene (3 mL) and tris(dibenzylideneacetone)-dipalladium (Pd2dba3) (2 mg, 0.002 mmol) were added under an argon atmosphere. The reaction mixture was stirred at 130 °C for 72 h. Upon cooling to room temperature, the reaction mixture was poured into methanol, and the precipitate was collected by filtration and subjected to Soxhlet extraction sequentially with acetone, hexanes, and chloroform. The chloroform solution was concentrated and precipitated in methanol. The precipitate was filtered and dried under vacuum to obtain a red-colored solid. Yield: 90 mg (82%). 1H-NMR spectrum is provided in ESI.† Elemental analysis: calculated for (C72H106N4O2S4)n (%): C 72.80, H 8.99, N 4.72; S 10.80 found (%): C 71.38, H 7.97, N 4.55, S 9.74.Synthesis of PPQ2T-TVT-24
This polymer was synthesized and purified using a similar procedure described for the synthesis of PPQ2T-TT-24, except that 1,2-bis(5-(trimethylstannyl)thiophen-2-yl)ethene (0.044 g, 0.09 mmol) was used as a comonomer in replacement of 2,5-bis(trimethylstannyl)thieno[3,2-b]thiophene. The product is a purple-colored solid. Yield: 96 mg (87%). 1H-NMR spectrum is provided in ESI.† Elemental analysis: calculated for (C76H110N4O2S4)n (%): C 73.62, H 8.94, N 4.52; S 10.34 found (%): C 72.50, H 7.93, N 4.18, S 9.63.OTFT device fabrication
Bottom-gate bottom-contact (BGBC) OTFT architecture was utilized for evaluating the polymers. An n++-doped Si wafer with a 300 nm thick SiO2 layer (dielectric) was used as the substrate. The gold source and drain electrodes were deposited onto the substrate by common photolithography and thermal evaporation methods. The substrate was then plasma treated and cleaned by ultra-sonication with acetone, isopropanol, and dried with nitrogen and baked at 120 °C for 1 min. The substrate was then cooled to room temperature, dried, and submerged in a DDTS solution (3% in toluene) for 20 min and then rinsed with toluene and dried with nitrogen. Polymer films with a thickness of ∼40 nm were deposited on the substrate by spin-coating a polymer solution in chloroform (5 mg mL−1) at 3000 rpm for 80 s and then annealed at an intended temperature for 20 min under nitrogen atmosphere in a glovebox. After annealing, the device performance was measured under nitrogen in the same glovebox and in the absence of light using an Agilent B2912A Precision source/measure unit. Devices characterized in the ambient air were annealed under nitrogen in the glovebox and then tested outside the glovebox in ambient room conditions with a relative humidity of 52%. Devices characterized in dry air and moist nitrogen were kept in a custom-made sealed chamber with electrical contacts for source, drain, and gate. Gases enter and leave via two ports. The charge carrier mobility (μ) and voltage threshold (VTH) in the saturation regime were calculated by using a slope obtained from the linear fitting of (IDS)1/2 against VGS according to the equation:57where IDS is the drain current, Ci is the insulator capacitance per unit area (∼11.6 nF cm−2), and VGS is the gate voltage, respectively. Device dimensions were 1 mm in width (W) and 30 μm in length (L). In the case for devices characterized in dry air and moist nitrogen device dimensions were 16.25 mm in width and 30 μm in length.
Acknowledgements
Financial support of this work by the Natural Sciences and Engineering Research Council (NSERC) of Canada (Discovery Grant # RGPIN-2016-04366) is acknowledged.Notes and references
- H. Sirringhaus, N. Tessler and R. H. Friend, Science, 1998, 280, 1741–1744 CrossRef CAS PubMed.
- C. D. Dimitrakopoulos and P. R. L. Malenfant, Adv. Mater., 2002, 14, 99–117 CrossRef CAS.
- S. R. Forrest, Nature, 2004, 428, 911–918 CrossRef CAS PubMed.
- H. Sirringhaus, Adv. Mater., 2005, 17, 2411–2425 CrossRef CAS.
- H. Yan, Z. Chen, Y. Zheng, C. Newman, J. R. Quinn, F. Dötz, M. Kastler and A. Facchetti, Nature, 2009, 457, 679–686 CrossRef CAS PubMed.
- A. Facchetti, Chem. Mater., 2011, 23, 733–758 CrossRef CAS.
- P. Sonar, S. P. Singh, Y. Li, Z.-E. Ooi, T. Ha, I. Wong, M. S. Soh and A. Dodabalapur, Energy Environ. Sci., 2011, 4, 2288–2296 CAS.
- A. Pron and M. Leclerc, Prog. Polym. Sci., 2013, 38, 1815–1831 CrossRef CAS.
- Y. Li, P. Sonar, L. Murphy and W. Hong, Energy Environ. Sci., 2013, 6, 1684–1710 CAS.
- X. Guo, M. Baumgarten and K. Müllen, Prog. Polym. Sci., 2013, 38, 1832–1908 CrossRef CAS.
- Y. He, W. Hong and Y. Li, J. Mater. Chem. C, 2014, 2, 8651–8661 RSC.
- C. Luo, A. K. K. Kyaw, L. A. Perez, S. Patel, M. Wang, B. Grimm, G. C. Bazan, E. J. Kramer and A. J. Heeger, Nano Lett., 2014, 14, 2764–2771 CrossRef CAS PubMed.
- X. Guo, A. Facchetti and T. J. Marks, Chem. Rev., 2014, 114, 8943–9021 CrossRef CAS PubMed.
- J. Quinn, C. Guo, B. Sun, A. Chan, Y. He, E. Jin and Y. Li, J. Mater. Chem. C, 2015, 3, 11937–11944 RSC.
- J. Quinn, E. Jin and Y. Li, Tetrahedron Lett., 2015, 56, 2280–2282 CrossRef CAS.
- M. J. Fray, J. P. Mathias, C. L. Nichols, Y. M. Po-Ba and H. Snow, Tetrahedron Lett., 2006, 47, 6365–6368 CrossRef CAS.
- U. a. Kshirsagar, S. B. Mhaske and N. P. Argade, Tetrahedron Lett., 2007, 48, 3243–3246 CrossRef CAS.
- Z. Zhang, X. Liang, X. Li, T. Song, Q. Chen and H. Sheng, Eur. J. Med. Chem., 2013, 69, 711–718 CrossRef CAS PubMed.
- S. G. Yerande, S. D. More, M. Bhandari, K. M. Newase, K. Khoury, K. Wang and A. Dömling, J. Heterocycl. Chem., 2014, 51, E358–E363 CrossRef CAS.
- M.-M. Zhang, L. Lu and X.-S. Wang, J. Heterocycl. Chem., 2014, 51, 1363–1368 CrossRef CAS.
- H. Chai, J. Li, L. Yang, H. Lu, Z. Qi and D. Shi, RSC Adv., 2014, 4, 44811–44814 RSC.
- I. Khan, A. Ibrar, W. Ahmed and A. Saeed, Eur. J. Med. Chem., 2015, 90, 124–169 CrossRef CAS PubMed.
- L.-P. Peng, S. Nagarajan, S. Rasheed and C.-H. Zhou, MedChemComm, 2015, 6, 222–229 RSC.
- Z. Chen, M. J. Lee, R. Shahid Ashraf, Y. Gu, S. Albert-Seifried, M. Meedom Nielsen, B. Schroeder, T. D. Anthopoulos, M. Heeney, I. McCulloch and H. Sirringhaus, Adv. Mater., 2012, 24, 647–652 CrossRef CAS PubMed.
- J.-R. Pouliot, B. Sun, M. Leduc, A. Najari, Y. Li and M. Leclerc, Polym. Chem., 2015, 6, 278–282 RSC.
- Z. Yi, S. Wang and Y. Liu, Adv. Mater., 2015, 27, 3589–3606 CrossRef CAS PubMed.
- J. Y. Back, H. Yu, I. Song, I. Kang, H. Ahn, T. J. Shin, S.-K. Kwon, J. H. Oh and Y.-H. Kim, Chem. Mater., 2015, 27, 1732–1739 CrossRef CAS.
- D. Khim, Y. R. Cheon, Y. Xu, W.-T. Park, S.-K. Kwon, Y.-Y. Noh and Y.-H. Kim, Chem. Mater., 2016, 28, 2287–2294 CrossRef CAS.
- H. Bronstein, Z. Chen, R. S. Ashraf, W. Zhang, J. Du, J. R. Durrant, P. Shakya Tuladhar, K. Song, S. E. Watkins, Y. Geerts, M. M. Wienk, R. A. J. Janssen, T. Anthopoulos, H. Sirringhaus, M. Heeney and I. McCulloch, J. Am. Chem. Soc., 2011, 133, 3272–3275 CrossRef CAS PubMed.
- I. Kang, H.-J. Yun, D. S. Chung, S.-K. Kwon and Y.-H. Kim, J. Am. Chem. Soc., 2013, 135, 14896–14899 CrossRef CAS PubMed.
- B. Sun, W. Hong, H. Aziz and Y. Li, Polym. Chem., 2015, 6, 938–945 RSC.
- M. J. Frisch, et al., Gaussian 09 Revision D.01, Gaussian, Inc., Wallingford, CT, USA, 2009. See ESI† for a full citation.
- H. Yamagata and F. C. Spano, J. Chem. Phys., 2012, 136, 184901–184914 CrossRef CAS PubMed.
- R. Noriega, J. Rivnay, K. Vandewal, F. P. V. Koch, N. Stingelin, P. Smith, M. F. Toney and A. Salleo, Nat. Mater., 2013, 12, 1038–1044 CrossRef CAS PubMed.
- F. C. Spano and C. Silva, Annu. Rev. Phys. Chem., 2014, 65, 477–500 CrossRef CAS PubMed.
- J. Pommerehne, H. Vestweber, W. Guss, R. F. Mahrt, H. Bässler, M. Porsch, J. Daub, H. Bassler, M. Porsch and J. Daub, Adv. Mater., 1995, 7, 551–554 CrossRef CAS.
- G. Kim, S.-J. Kang, G. K. Dutta, Y.-K. Han, T. J. Shin, Y.-Y. Noh and C. Yang, J. Am. Chem. Soc., 2014, 136, 9477–9483 CrossRef CAS PubMed.
- S. Chen, B. Sun, W. Hong, H. Aziz, Y. Meng and Y. Li, J. Mater. Chem. C, 2014, 2, 2183–2190 RSC.
- D. M. de Leeuw, M. M. J. Simenon, A. R. Brown and R. E. F. Einerhand, Synth. Met., 1997, 87, 53–59 CrossRef CAS.
- T. D. Anthopoulos, G. C. Anyfantis, G. C. Papavassiliou and D. M. de Leeuw, Appl. Phys. Lett., 2007, 90, 122105 CrossRef.
- H. T. Nicolai, M. Kuik, G. a. H. Wetzelaer, B. de Boer, C. Campbell, C. Risko, J. L. Brédas and P. W. M. Blom, Nat. Mater., 2012, 11, 882–887 CrossRef CAS PubMed.
- A. Virkar, S. Mannsfeld, J. H. Oh, M. F. Toney, Y. H. Tan, G. Liu, J. C. Scott, R. Miller and Z. Bao, Adv. Funct. Mater., 2009, 19, 1962–1970 CrossRef CAS.
- Y. Lei, B. Wu, W.-K. E. Chan, F. Zhu and B. S. Ong, J. Mater. Chem. C, 2015, 3, 12267–12272 RSC.
- B. Park, Thin Solid Films, 2015, 598, 141–148 CrossRef.
- X. Yan, H. Wang and D. Yan, Thin Solid Films, 2006, 515, 2655–2658 CrossRef CAS.
- R. Schmidt, J. H. Oh, Y.-S. Sun, M. Deppisch, A.-M. Krause, K. Radacki, H. Braunschweig, M. Könemann, P. Erk, Z. Bao and F. Würthner, J. Am. Chem. Soc., 2009, 131, 6215–6228 CrossRef CAS PubMed.
- J. H. Oh, S. Suraru, W.-Y. Lee, M. Könemann, H. W. Höffken, C. Röger, R. Schmidt, Y. Chung, W.-C. Chen, F. Würthner and Z. Bao, Adv. Funct. Mater., 2010, 20, 2148–2156 CrossRef CAS.
- X. Guo, R. P. Ortiz, Y. Zheng, M.-G. Kim, S. Zhang, Y. Hu, G. Lu, A. Facchetti and T. J. Marks, J. Am. Chem. Soc., 2011, 133, 13685–13697 CrossRef CAS PubMed.
- T. Lei, Y. Cao, Y. Fan, C.-J. Liu, S.-C. Yuan and J. Pei, J. Am. Chem. Soc., 2011, 133, 6099–6101 CrossRef CAS PubMed.
- S. Lee, D. C. Borrelli and K. K. Gleason, Org. Electron., 2016, 33, 253–262 CrossRef CAS.
- L. Guo, J. Quinn, J. Wang, C. Guo, X. Li, J. Wang and Y. Li, Dyes Pigm., 2016, 132, 329–335 CrossRef CAS.
- G. Lu, H. Usta, C. Risko, L. Wang, A. Facchetti, M. A. Ratner and T. J. Marks, J. Am. Chem. Soc., 2008, 130, 7670–7685 CrossRef CAS PubMed.
- Y. Qiu, Y. Hu, G. Dong, L. Wang, J. Xie and Y. Ma, Appl. Phys. Lett., 2003, 83, 1644–1646 CrossRef CAS.
- Z.-T. Zhu, J. T. Mason, R. Dieckmann and G. G. Malliaras, Appl. Phys. Lett., 2002, 81, 4643–4645 CrossRef CAS.
- R. Ye, M. Baba, K. Suzuki, Y. Ohishi and K. Mori, Thin Solid Films, 2004, 464–465, 437–440 CrossRef CAS.
- H. E. Gottlieb, V. Kotlyar and A. Nudelman, J. Org. Chem., 1997, 62, 7512–7515 CrossRef CAS PubMed.
- G. Horowitz, Adv. Mater., 1998, 10, 365–377 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of computer simulations, TGA, DSC, and additional OTFT data. See DOI: 10.1039/c6ra17595c |
| This journal is © The Royal Society of Chemistry 2016 |


