Ultra-low dielectric constant materials with hydrophobic property derived from polyhedral oligomeric silsequioxane (POSS) and perfluoro-aromatics
Abstract
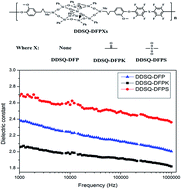
A series of poly(arylene ether)s were synthesized with a diphenol POSS (2OH-DDSQ) and perfluoro-aromatics including decafluorobiphenyl, decaflurobenzophenone and decafluorodiphenyl sulfone via a two-step nucleophilic aromatic substitution polymerization. The chemical structures of the polymers were confirmed by 19F-NMR and 1H-NMR. All the polymers exhibit not only low dielectric constants in the range of 1.85–2.37 at 1 MHz at room temperature but also low dielectric losses, hydrophobic properties, low water uptake and good thermal properties. In particular, the DDSQ-DFPK film showed the ultra-low dielectric constant of 1.83 at 1 MHz. With increasing polarity of the perfluoro-aromatics, the water contact angles decrease from 108° to 102°.

 Please wait while we load your content...
Please wait while we load your content...