Toward clean suspended CVD graphene†
Alexander Yulaevabc,
Guangjun Chengd,
Angela R. Hight Walkerd,
Ivan V. Vlassiouke,
Alline Myersa,
Marina S. Leite*bf and
Andrei Kolmakov*a
aCenter for Nanoscale Science and Technology, NIST, Gaithersburg, MD 20899, USA. E-mail: andrei.kolmakov@nist.gov
bDepartment of Materials Science and Engineering, University of Maryland, College Park, MD 20742, USA. E-mail: mleite@umd.edu
cMaryland NanoCenter, University of Maryland, College Park, MD 20742, USA
dPhysical Measurement Laboratory, NIST, Gaithersburg, MD 20899, USA
eOak Ridge National Laboratory, Oak Ridge, TN 37831, USA
fInstitute for Research in Electronics and Applied Physics, University of Maryland, College Park, 20742, USA
First published on 26th August 2016
Abstract
The application of suspended graphene as electron transparent supporting media in electron microscopy, vacuum electronics, and micromechanical devices requires the least destructive and maximally clean transfer from their original growth substrate to the target of interest. Here, we use thermally evaporated anthracene films as the sacrificial layer for graphene transfer onto an arbitrary substrate. We show that clean suspended graphene can be achieved via desorbing the anthracene layer at temperatures in the 100 °C to 150 °C range, followed by two sequential annealing steps for the final cleaning, using a Pt catalyst and activated carbon. The cleanliness of the suspended graphene membranes was analyzed employing the high surface sensitivity of low energy scanning electron microscopy and X-ray photoelectron spectroscopy. A quantitative comparison with two other commonly used transfer methods revealed the superiority of the anthracene approach to obtain a larger area of clean, suspended CVD graphene. Our graphene transfer method based on anthracene paves the way for integrating cleaner graphene in various types of complex devices, including the ones that are heat and humidity sensitive.
Introduction
Ultra-thin and clean suspended graphene (Gr) membranes have been applied in a variety of micromechanical devices,1 sensors,2 and vacuum electronics;3 as supporting media for high-resolution transmission electron microscopy (HRTEM),4 and as electron transparent windows in ambient pressure photoelectron spectroscopy and microscopy.5 The mechanical exfoliation of the graphite flakes remains to be the cleanest method to prepare such devices but it is low yield and labor intensive. Alternatively, ultrathin membranes assembled from interlocked chemically exfoliated graphene (graphene oxide) flakes or platelets are simple to fabricate,6 however, they possess a large amount of defects and reactive functional groups. The latter, being advantageous for the development of new composite materials7 via graphene functionalization, can lead to undesirable alteration of the physical and chemical properties of the membrane devices. On the other hand, the growth of graphene via chemical vapor deposition (CVD) on copper or nickel substrates is a well-developed, large-scale, and high-yield method of graphene production with large single crystal domain size.8 In order to transfer graphene from copper or nickel foils onto a target substrate, multiple approaches have been implemented, which can be roughly classified into two groups: “wet” and “dry” methods, depending on whether any liquid is involved during the final stage of the graphene layer transfer. For suspended graphene devices, wet transfer methods via a sacrificial layer9–11 or direct transfer approaches using capillary action of solvent droplets12 (e.g. isopropyl alcohol (IPA)) as an adhesion promoter are commonly used. The former method usually relies on a polymethyl methacrylate (PMMA) layer spin-coated over the Gr–metal substrate, followed by etching of the metal and transferring the PMMA/Gr onto a target substrate. After the transfer, the PMMA layer is dissolved in solvents, or removed by annealing in vacuum13 or under reducing conditions.14 Recently, an alternative polymer scaffolds have been used instead of PMMA, which do not require an additional annealing after the polymers are dissolved.15 However, some of the copper etchants, such as ammonium persulfate, can promote a crosslinking in polymers, resulting in a high concentration of residues on the graphene layer.16 Alternatively, the direct transfer method is based on an adhesion between a Gr/substrate stack and a flexible perforated carbon membrane induced by capillary forces of a drying solvent such as IPA.12 In this case, the growth substrate is etched away, and, after rinsing in water and drying, a suspended, high-quality membrane is obtained. Other wet methods of graphene transfer include the soak-and-peel,17 bubbling transfer,18 and electrochemical delamination (see ref. 19–21 for a detailed description of each approach).Dry transfer of graphene is necessary when the target substrate is reactive or sensitive to moisture. Solvent-free methods to transfer CVD-grown Gr employ polydimethylsiloxane (PDMS) stamps,22 thermal release tape,23 thermal decomposition of PMMA in forming gas,9 etc.
The application of graphene in electron or scanning probe microscopies, in microelectromechanical systems (MEMS)/electronic devices and many other fields requires the ultimate surface cleanliness of the suspended membrane. However, all the aforementioned transfer methods unavoidably contaminate the graphene surface, requiring a sequential step of rigorous cleaning. The direct comparison of the reported cleaning methods is hard to conduct due to the variety of transfer conditions and characterization techniques used. Therefore, a systematic and quantitative comparison of the most commonly used methods is essential to determine the most effective one.
Here, we demonstrate a novel ultraclean method for the transfer of CVD-grown graphene utilizing an anthracene film as a sacrificial layer. Different from higher molecular mass polycyclic aromatic hydrocarbons (PAHs) that strongly interact with graphene, an anthracene film can easily sublime at moderate temperature (<150 °C),24 thus preventing structural changes of the graphene and a sensitive target substrate. We also perform a comparative cleanliness analysis of our approach with two widely used transfer methods: (i) direct transfer by IPA12 and (ii) PMMA-based transfer.9 The samples transferred via all three aforementioned protocols were subjected to the same two step cleaning procedure after the transfer: annealing in the presence of platinum catalyst25 followed by annealing in activated carbon.26 The cleanliness of the resultant membranes was examined with techniques highly sensitive to the surface contamination and defects: low-voltage (1 keV) scanning electron microscopy (LVSEM), X-ray photoelectron spectroscopy (XPS), and transmission electron microscopy (TEM). We show that graphene transferred by anthracene is consistently cleaner and less defective than samples prepared using other methods.
Experimental procedures
Sample preparation and transfer using anthracene
Graphene was grown from a methane gas precursor on a copper substrate using the standard CVD method, which is described in details elsewhere.8,27,28 Briefly, electropolished in phosphoric acid 125 μm thick copper foils were loaded into atmospheric pressure CVD reactor and annealed at 1065 °C under the flow of 2.5% H2 in Ar for 30 min. Graphene growth was performed by addition of methane with a gradual increase of concentration from 10 to 20 and then to 40 ppm for 30 min increments. After cooling down to room temperature, a monolayer of graphene with less than 5% fraction of the hexagon-shaped bilayer was formed on both sides of the copper foil.An anthracene film with an average thickness of 14 μm ± 7 μm was thermally evaporated onto as-grown graphene on a copper foil in vacuum (≈10−3 Pa) (Fig. 1a(ii)). Dense and mechanically stable, quasi-amorphous films were obtained if the deposition was conducted onto a pre-cooled (≈−20 °C) Gr/substrate in vacuum. Note that low temperature of a substrate is essential to increase the density of the nucleation sites for anthracene (Fig. S1†). After the deposition onto the pre-cooled sample (Fig. 1a(ii)), the copper substrate was etched in aqueous ammonium persulfate solution (APS) (Fig. 1a(iii)). The Gr-anthracene stack was then rinsed in distilled water, and transferred directly to the TEM mesh for inspection (Fig. 1a(iv)). After annealing at 120 °C for 40 min (Fig. 1a(v)), the anthracene film sublimed, and only the graphene layer was left on the target substrate (Fig. 1a(vi)). Besides the low temperature sublimation of anthracene, the other advantage of this PAH is its high fluorescence yield under ultraviolet (UV) light irradiation (Fig. 1b and c), which helps visualize the anthracene during the transfer process and track residues left on graphene after annealing (Fig. 1b and c).
Comparative analysis
Three sets of graphene membrane samples transferred using (i) PMMA as a sacrificial layer, (ii) IPA droplet capillary adhesion (so called “direct transfer”), and (iii) anthracene as a sacrificial layer were subjected to the same set of cleaning treatments and characterizations to compare and identify the least contaminated product.The sample fabrication for the comparative analysis is schematically illustrated in Fig. 2. First, TEM grids made of gold with a perforated, 20 nm thick carbon mesh (2 μm hole diameter) were half-coated with a 10 nm platinum layer to compare the effect of the platinum catalysis on the surface purity (Fig. 2a) on the same substrate. Second, CVD-Gr films were transferred onto the grids using the three methods depicted in Fig. 2b. All the samples concurrently underwent first the catalytic cleaning (180 °C, 30 min) on a hot plate in ambient air, followed by an activated carbon cleaning procedure step (210 °C, 90 min, rate 5 °C min−1) in an oven. We used LVSEM to image all samples immediately as transferred, after the platinum-catalysis treatment, and, ultimately, after the cleaning in activated carbon. For LVSEM imaging, all samples have been mounted on a graphite specimen stub to minimize the background signal formed by spurious secondary electrons. The same contrast/brightness adjustments were maintained during all three LVSEM sessions to quantitatively compare results of each cleaning step. To maximize surface sensitivity of the SEM to impurities, a low-energy electron beam (1 keV) in combination with through-the-lens secondary electrons detector, and short working distance (3 mm) were used. All samples have been studied by TEM at 300 keV electron beam energy.
XPS spectra of graphene samples were collected at ≈3 × 10−7 Pa in ultra-high vacuum (UHV) chamber equipped with a 125 mm radius hemispherical electron energy analyzer operating with an emission angle of 54°. The monochromatic Al Kα (1486.6 eV) X-ray source was used for the XPS measurements. The analyzer was working at the constant pass energy Ep = 13.6 eV and slit sizes offering an experimental energy resolution 0.55 eV. The XPS peaks of graphene were deconvoluted using mixed asymmetric Gaussian–Lorentzian line shapes after a Shirley background subtraction.
Results and discussion
The resulting suspended graphene obtained by the anthracene-based method demonstrates high yield of successful (with no holes) coverage over the perforated structure, ≈95% (Fig. S3†), comparable with the best results obtained using the direct transfer by IPA drop and PMMA sacrificial layer. The quality of the as transferred graphene was evaluated using Raman spectroscopy via measuring relative intensity of the D-peak and G/D ratio (Fig. S4†). Different from commonly used high voltage (5 kV to 15 kV) SEM imaging, we employed low electron beam energies (0.5 keV to 1 keV) in combination with a true secondary electron (SE) detector to monitor the contaminants evolution upon different cleaning procedures. The enhanced electron interaction cross-section and surface sensitivity of such imaging conditions are advantageous for discriminating between clean and contaminated regions on the Gr layer.29,30SEM analysis
According to the semi-empirical law for the SE emission from carbon,31 the dependence of SE yield on electron beam energy EPE has a maximum at 400 eV and decreases with electron beam energy due to the decrease of the stopping power. The dependence of SE yield δ on membrane thickness d and EPE, can be evaluated31 as:where ε, λ, and R stand for the effective energy to produce SE, the effective SE escape depth, and the penetration depth of the incident electron, respectively. For a carbon membrane with ε = 80 eV,31 λ = 2.5 nm, and R = 7 nm, the formula yields δ = 0.1, 0.2, and 0.3 for 1, 2, and 3 layer thick graphene, respectively, implying that a clean, suspended, single Gr layer will be distinguishable from any additional carbon-containing residual layers. For our experiments we used 1 keV electron beam energy to reduce electron induced carbon contamination.
Fig. 3 demonstrates the effectiveness of low-voltage SEM (LVSEM) to evaluate the cleanliness of suspended membrane. The panel (a) in Fig. 3 depicts the model consisting of 20 nm thick carbon mesh covered with 10 nm Pt and one layer of graphene as in the real sample. Four carbon pads of 1, 2, 5, and 10 layer thicknesses and an open orifice mimic different levels of contamination and a tear in graphene layer, respectively. Fig. 3b shows the corresponding Monte Carlo simulated32 SE images, where the grayscale refers to the number of SEs collected per 1000 primary electrons. According to the simulation, the impurity pads are clearly distinguishable from a pristine single-layer graphene if a low energy (<1 keV) primary electron beam is used as the SE yield increases with impurity thickness. The experimental results qualitatively corroborate with our simulations (Fig. 3c and d). As the energy of the electron beam increases from 1 keV to 3 keV, the overall SE yield from the membrane diminishes due to the reduction in the inelastic interaction cross-section. As a result, the contrast between clean, contaminated graphene and void areas decreases (Fig. 3e) and the carbon membrane appears to be more transparent and cleaner.
LVSEM images of suspended graphene on a perforated carbon film with Pt (10 nm) film transferred using PMMA (top: a–c), IPA (center: d–f), and an anthracene sacrificial layer (bottom: g–i) are shown in Fig. 4. All samples demonstrate visible contamination before cleaning, which is noted as bright spots and lighter color corrugated regions. In the case of PMMA, the typical residues are left from incomplete scission of PMMA bonds during cleaning steps.16
Contamination of graphene transferred by anthracene and IPA methods is mainly due to hydrocarbons; in particular, (–CH2–) and (–CH3) groups of hydrocarbons accumulated on the surface have been routinely detected after samples were exposed to air.33
A comparison between the different transfer methods was performed by imaging samples after each sequential cleaning step by SEM, see Fig. 4. The images in the left, central, and right columns show suspended graphene before cleaning, after Pt catalytic cleaning at 180 °C, and then annealing in activated carbon, respectively. Each image contains the regions with a suspended membrane (dark circle in the center), graphene over Pt-covered carbon mesh (peripheral brightest area), and open holes (the darkest area). SEM images of moderately cleaned CVD-Gr revealed the accumulation of the contaminants in elongated strips (Fig. 4b and h). The origin of such impurity distribution is attributed to stronger affinity of the impurity molecules to graphene point and extended defects,34,35 as well as to wrinkles, which are common in CVD-grown graphene and occur as a result of compressive strain in as-grown carbon monolayer.36 According to the set of SEM images of differently prepared samples after the aforementioned treatments, PMMA and anthracene methods demonstrate fewer residues on a carbon monolayer after two sequential cleaning steps compared to the direct transfer using IPA. The evolution of the residue concentration with a cleaning sequence can be illustrated using PMMA transferred graphene as an example. As transferred (before any additional cleaning) membrane routinely contains polymer contaminants seen as multiple bright spots and network of gray patches over the membrane (Fig. 4a). The cleanliness of the suspended membranes at a microscale level can be addressed more quantitatively using the gray scale values (GSV) of SEM images, implying that the GSV is proportional to the total SE signal from the corresponding area. To conduct such measurements, the SEM detector was tuned to the same fixed values of gain and offset for entire set of measurements. The results of this analysis for all three transfer methods and samples with and without pre-deposited Pt catalyst are summarized in Fig. 5. The vertical axis values correspond to SE signal of the imaged graphene with respect to the open hole. Therefore, the cleanest and the most transparent sample will have the SE signal approaching zero.
After annealing the samples on a hot plate, the overall SE signal from all samples decreases since the large partition of the PMMA remnants become decomposed by Pt catalysis. During this process, end-chain PMMA dissociation is initiated, and the polymer degrades into monomers that can sublime.25 Interestingly, the PMMA decomposition can be observed even a few microns away from the Pt catalyst (Fig. S5†). We assign this extended catalytic action to a spillover effect when dissociated reactive species such as hydrogen and/or oxygen migrate from Pt to PMMA residues.37 As a result, the transparency of graphene after annealing in the presence of Pt catalyst increases by ≈50% (Fig. 5 right semi-plane). The electron transparency of the membrane without Pt (left semi-plane) also improves after the same treatment, however, only by ≈30%, which confirms the noticeable contribution of catalytic activation and spillover effect.
As can be seen from Fig. 5 (blue dots), Pt catalysis is effective not only for PMMA decomposition, but also for cleaning of the membrane from hydrocarbon contaminants after IPA transfer. The latter results in ≈50% cleaning effect after the treatment (compare with only ≈20% cleaning without Pt). The similar cleaning trend can be observed for graphene transferred by anthracene yielding the cleaning effect of ≈50% after thermal desorption of contaminants.
The cleaning effect of activated carbon with high degree of microporosity, which provides high surface area, is based on its adsorptive capacity. Upon thermal activation, the contaminants left on a graphene surface randomly diffuse until they become adsorbed and trapped by high surface area activated carbon particle. This second step of cleaning significantly improves the cleanliness of the PMMA and anthracene transferred graphene. This is not a case, though, for impurities left from IPA-based transfer. The cleaning effect is most pronounced for the sample with anthracene impurities annealed in the presence of a Pt catalyst and activated carbon. After the treatment, the transparency of the suspended membrane increased up to ≈65% compared to as transferred graphene (Fig. 5, green dots). To summarize, the combination of two aforementioned cleaning steps provides an efficient recipe to clean Gr, independent of whether PMMA or anthracene impurities were present on the sample.
It is necessary to note that electron beam induced carbon contamination can often be observed during SEM inspection of the suspended membrane.38 This effect is particularly pronounced when the sample has hydrocarbon impurities and, therefore, can be used as an additional tool to evaluate graphene purity before and after the transfer. Low energy secondary electrons are mainly responsible for the dissociation of surface hydrocarbons and the buildup of the carbon deposit on a membrane.16 Interestingly, both as transferred and partially cleaned (cleaning step I, Fig. 5) samples have demonstrated prominent contamination buildup during SEM imaging at room temperature after 15 s of irradiation (Fig. S6†). However, the carbon contamination became negligible after samples were cleaned in activated carbon, indicating that the source of the hydrocarbons are the surface residues left on samples.
XPS analysis
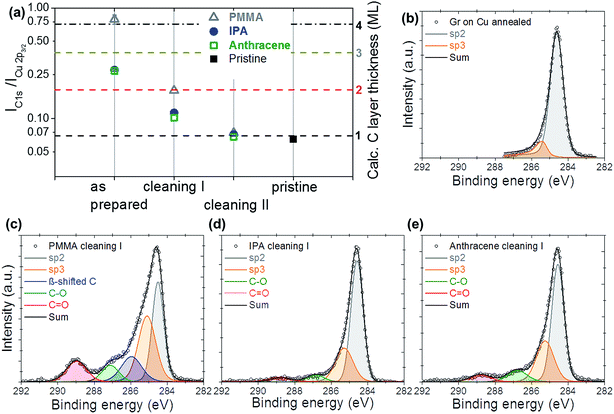
The microscopy results have been complemented with XPS analysis of graphene purity. For that, CVD Gr/Cu samples underwent the same set of aforementioned cleaning procedures. In particular, PMMA-covered/Gr/Cu, anthracene-covered/Gr/Cu, and IPA-immersed/Gr/Cu samples were prepared first using the standard procedures: removing the sacrificial layers by acetone, thermal sublimation, and drying, respectively. The samples then were first annealed in air (cleaning I) followed by annealing in activated carbon (cleaning II). The effective thickness of the overlay can be evaluated from the corresponding attenuation of the XPS substrate signal by carbonaceous contaminants layer.39 For that, the cumulative intensity ratios of C 1s peak to attenuated Cu 2p3/2 peak were measured at the same spot after the sample preparation and after each cleaning procedure (Fig. 6a). These data were compared to Cu 2p3/2 peak attenuation test of as grown and vacuum annealed pristine Gr/Cu sample considered to be ultimately clean (black square in the Fig. 6a). The SESSA algorithm40 was used to compare experimental Cu 2p3/2 peak attenuation data with theoretical predictions for 1 to 4 carbon monolayers (ML) (Fig. 6a). The XPS-assessed effective thickness of contaminants corroborates well with the trend observed via electron microscopy. In particular, as prepared samples exhibit the highest degree of contamination reaching almost four effective monolayers of impurities in the case of a PMMA-based sacrificial layer. The cleanliness of the samples improves progressively with sequential cleaning. Similar to the SEM observations above, anthracene and IPA introduced the least amount of contaminations at the graphene surface, and the final cleanliness approaches the quality of as grown and vacuum annealed CVD Gr/Cu sample, matching the theoretically predicted C 1s/Cu 2p3/2 ratio for 1 ML of carbon on copper.More information on chemical nature of the contaminants can be obtained from the C 1s peak shape evolution upon the cleaning treatments. Fig. 6b depicts the C 1s spectrum of as grown and vacuum annealed (10−7 Pa, 250 °C, 2 hours) Gr/Cu sample, that we consider ultimately clean graphene. The spectrum contains dominating sp2 graphene component and minor contribution from sp3 impurities separated by ≈0.75 eV. Fig. 6c–e show XPS spectra of PMMA, IPA, and anthracene transferred samples after cleaning step I. This intermediate cleaning of the PMMA sample did not completely removed a polymer as can be seen from the prominent contribution from PMMA related peaks41 compared to sp2 signal from graphene (Fig. 6c). On the other hand, the IPA and anthracene samples exhibited dominating graphene (sp2) contribution and traces of carboxyl (binding energy (BE) ≈ 289 eV), methoxy (BE ≈ 287 eV) groups, and sp3 carbon (Fig. 6d and e). The overall trend observed in XPS measurements agrees with SEM and Raman results and shows significant reduction of impurity peaks upon cleaning in favor of pure sp2 feature.
TEM study
Finally, the samples have also been examined using TEM to compare the quality of the resultant membranes at the nanoscale.Fig. 7a and b show TEM images of the PMMA transferred graphene after final cleaning in activated carbon taken from the areas not affected by Pt catalyst (panel a) and areas in proximity to Pt catalyst (panel b). The membranes have the domains of pristine graphene and network of contaminants. The presence of the Pt catalyst results in enlargement of the area of pristine graphene, which can be as large as ≈2 × 103 nm2. The cleaning effect is even more pronounced for anthracene impurities (compare Fig. 7c and d). The combined Pt and activated carbon cleaning results in the appearance of very large patches of clean graphene with a typical area ≈ 2 × 104 nm2, which is comparable to or even better than previously reported results.42
 | ||
| Fig. 7 TEM images of graphene transferred by PMMA (a and b) and anthracene (c and d) onto a carbon mesh and treated thermally in the presence (b and d) and without (a and c) Pt catalyst. Insets are FFTs of HR TEM images (21 nm × 21 nm) obtained from representative regions for each method (see also Fig. S7†). | ||
Conclusions
In conclusion, we present a new method of clean CVD-Gr transfer using anthracene as a sacrificial layer. It should not be seen a universal or simplest method but rather suitable for specific applications where graphene coverage is required over the chemically reactive and temperature sensitive substrates. The advantage of this approach is the dry removal of the sacrificial layer at temperatures below 150 °C, which often is a requirement for the aforementioned systems. Using high surface sensitivity of LVSEM and XPS, we compare the cleanliness of the suspended membranes transferred by different methods with the same cleaning procedures. SEM, XPS and TEM studies demonstrated the advantage of the anthracene method in combination with annealing in air in the presence of Pt catalyst followed by annealing in activated carbon to achieve a cleaner CVD-grown graphene. Note, the thermal treatment of the graphene in activated carbon has a potential drawback: after cleaning, a small amount of activated carbon dust particles adheres to the sample. Therefore, whether cleaning in activated carbon should be applied depends on the particular graphene application. We envision that our approach may be suitable in applications where dry and clean transfer protocols are required.Author contributions
AK, AY, and MSL conceived and designed the experiments; AY, AK and GC performed the experiments and analyzed the data; IVV produced graphene samples; GC, AM and AHW characterized the samples; AY, AK, MSL, and AHW co-wrote the paper.Acknowledgements
This work has been conducted at CNST, NIST using CNST NanoFab facilities and was supported in part by the NIST-CNST/UMD-IREAP Cooperative Agreement. MSL thanks the 2014 Minta Martin Award at UMD. The discussions and feedback from Dr I. Levin and Dr N. Zhitenev are greatly appreciated.References
- J. S. Bunch, A. M. Van Der Zande, S. S. Verbridge, I. W. Frank, D. M. Tanenbaum, J. M. Parpia, H. G. Craighead and P. L. McEuen, Science, 2007, 315, 490–493 CrossRef CAS PubMed.
- F. Schedin, A. Geim, S. Morozov, E. Hill, P. Blake, M. Katsnelson and K. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- J.-N. Longchamp, T. Latychevskaia, C. Escher and H.-W. Fink, Appl. Phys. Lett., 2012, 101, 113117 CrossRef.
- J. M. Yuk, J. Park, P. Ercius, K. Kim, D. J. Hellebusch, M. F. Crommie, J. Y. Lee, A. Zettl and A. P. Alivisatos, Science, 2012, 336, 61–64 CrossRef CAS PubMed.
- J. Kraus, R. Reichelt, S. Guenther, L. Gregoratti, M. Amati, M. Kiskinova, A. Yulaev, I. Vlassiouk and A. Kolmakov, Nanoscale, 2014, 6, 14394–14403 RSC.
- M. Krueger, S. Berg, D. A. Stone, E. Strelcov, D. A. Dikin, J. Kim, L. J. Cote, J. Huang and A. Kolmakov, ACS Nano, 2011, 5, 10047–10054 CrossRef CAS PubMed.
- J. Gu, X. Yang, Z. Lv, N. Li, C. Liang and Q. Zhang, Int. J. Heat Mass Transfer, 2016, 92, 15–22 CrossRef CAS.
- X. Li, C. W. Magnuson, A. Venugopal, R. M. Tromp, J. B. Hannon, E. M. Vogel, L. Colombo and R. S. Ruoff, J. Am. Chem. Soc., 2011, 133, 2816–2819 CrossRef CAS PubMed.
- J. W. Suk, A. Kitt, C. W. Magnuson, Y. Hao, S. Ahmed, J. An, A. K. Swan, B. B. Goldberg and R. S. Ruoff, ACS Nano, 2011, 5, 6916–6924 CrossRef CAS PubMed.
- A. Reina, H. Son, L. Jiao, B. Fan, M. S. Dresselhaus, Z. Liu and J. Kong, J. Phys. Chem. C, 2008, 112, 17741–17744 CAS.
- C. Dean, A. Young, I. Meric, C. Lee, L. Wang, S. Sorgenfrei, K. Watanabe, T. Taniguchi, P. Kim and K. Shepard, Nat. Nanotechnol., 2010, 5, 722–726 CrossRef CAS PubMed.
- W. Regan, N. Alem, B. Alemán, B. Geng, Ç. Girit, L. Maserati, F. Wang, M. Crommie and A. Zettl, Appl. Phys. Lett., 2010, 96, 113102 CrossRef.
- Z. Cheng, Q. Zhou, C. Wang, Q. Li, C. Wang and Y. Fang, Nano Lett., 2011, 11, 767–771 CrossRef CAS PubMed.
- M. Ishigami, J. Chen, W. Cullen, M. Fuhrer and E. Williams, Nano Lett., 2007, 7, 1643–1648 CrossRef CAS PubMed.
- J. D. Wood, G. P. Doidge, E. A. Carrion, J. C. Koepke, J. A. Kaitz, I. Datye, A. Behnam, J. Hewaparakrama, B. Aruin and Y. Chen, Nanotechnology, 2015, 26, 055302 CrossRef PubMed.
- Y.-C. Lin, C.-C. Lu, C.-H. Yeh, C. Jin, K. Suenaga and P.-W. Chiu, Nano Lett., 2011, 12, 414–419 CrossRef PubMed.
- P. Gupta, P. D. Dongare, S. Grover, S. Dubey, H. Mamgain, A. Bhattacharya and M. M. Deshmukh, Sci. Rep., 2014, 4, 3882 Search PubMed.
- L. Gao, W. Ren, H. Xu, L. Jin, Z. Wang, T. Ma, L.-P. Ma, Z. Zhang, Q. Fu and L.-M. Peng, Nat. Commun., 2012, 3, 699 CrossRef PubMed.
- J. Kang, D. Shin, S. Bae and B. H. Hong, Nanoscale, 2012, 4, 5527–5537 RSC.
- A. V. Zaretski and D. J. Lipomi, Nanoscale, 2015, 7, 9963–9969 RSC.
- C. T. Cherian, F. Giustiniano, I. Martin-Fernandez, H. Andersen, J. Balakrishnan and B. Özyilmaz, Small, 2015, 11, 189–194 CrossRef CAS PubMed.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi and B. H. Hong, Nature, 2009, 457, 706–710 CrossRef CAS PubMed.
- S. Bae, H. Kim, Y. Lee, X. Xu, J.-S. Park, Y. Zheng, J. Balakrishnan, T. Lei, H. R. Kim and Y. I. Song, Nat. Nanotechnol., 2010, 5, 574–578 CrossRef CAS PubMed.
- V. Oja and E. M. Suuberg, J. Chem. Eng. Data, 1998, 43, 486–492 CrossRef CAS.
- J.-N. Longchamp, C. Escher and H.-W. Fink, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.--Process., Meas., Phenom., 2013, 31, 020605 CrossRef.
- G. Algara-Siller, O. Lehtinen, A. Turchanin and U. Kaiser, Appl. Phys. Lett., 2014, 104, 153115 CrossRef.
- X. Li, W. Cai, J. An, S. Kim, J. Nah, D. Yang, R. Piner, A. Velamakanni, I. Jung and E. Tutuc, Science, 2009, 324, 1312–1314 CrossRef CAS PubMed.
- I. Vlassiouk, M. Regmi, P. Fulvio, S. Dai, P. Datskos, G. Eres and S. Smirnov, ACS Nano, 2011, 5, 6069–6076 CrossRef CAS PubMed.
- E. Mikmeková, H. Bouyanfif, M. Lejeune, I. Müllerová, M. Hovorka, M. Unčovský and L. Frank, J. Microsc., 2013, 251, 123–127 CrossRef PubMed.
- G. Cheng, I. Calizo and A. R. H. Walker, Carbon, 2015, 81, 678–687 CrossRef CAS.
- Y. Lin and D. C. Joy, Surf. Interface Anal., 2005, 37, 895–900 CrossRef CAS.
- H. Demers, N. Poirier-Demers, A. R. Couture, D. Joly, M. Guilmain, N. de Jonge and D. Drouin, Scanning, 2011, 33, 135–146 CrossRef CAS PubMed.
- Z. Li, Y. Wang, A. Kozbial, G. Shenoy, F. Zhou, R. McGinley, P. Ireland, B. Morganstein, A. Kunkel and S. P. Surwade, Nat. Mater., 2013, 12, 925–931 CrossRef CAS PubMed.
- O. Cretu, A. V. Krasheninnikov, J. A. Rodríguez-Manzo, L. Sun, R. M. Nieminen and F. Banhart, Phys. Rev. Lett., 2010, 105, 196102 CrossRef PubMed.
- P. Y. Huang, C. S. Ruiz-Vargas, A. M. van der Zande, W. S. Whitney, M. P. Levendorf, J. W. Kevek, S. Garg, J. S. Alden, C. J. Hustedt and Y. Zhu, Nature, 2011, 469, 389–392 CrossRef CAS PubMed.
- X. Li, Y. Zhu, W. Cai, M. Borysiak, B. Han, D. Chen, R. D. Piner, L. Colombo and R. S. Ruoff, Nano Lett., 2009, 9, 4359–4363 CrossRef CAS PubMed.
- A. Pirkle, J. Chan, A. Venugopal, D. Hinojos, C. Magnuson, S. McDonnell, L. Colombo, E. Vogel, R. Ruoff and R. Wallace, Appl. Phys. Lett., 2011, 99, 122108 CrossRef.
- M. Her, R. Beams and L. Novotny, Phys. Lett. A, 2013, 377, 1455–1458 CrossRef CAS.
- C. Fadley, R. Baird, W. Siekhaus, T. Novakov and S. Bergström, J. Electron Spectrosc. Relat. Phenom., 1974, 4, 93–137 CrossRef CAS.
- W. Smekal, W. S. Werner and C. J. Powell, Surf. Interface Anal., 2005, 37, 1059–1067 CrossRef CAS.
- G. Beamson and D. Briggs, High resolution XPS of organic polymers, Wiley, 1992 Search PubMed.
- R. Zan, U. Bangert, Q. Ramasse and K. S. Novoselov, J. Phys. Chem. Lett., 2012, 3, 953–958 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17360h |
| This journal is © The Royal Society of Chemistry 2016 |